59+ Classical Solar System Atomic Model
59+ Classical Solar System Atomic Model. 12/07/2013 · the most instantly recognizable image of an atom resembles a miniature solar system with the concentric electron paths forming the planetary orbits and the nucleus at the centre like the sun. 08/07/2020 · with his classic, alpha ray scattering experiment rutherford was able to suggest a possible model for the atom.
Nejchladnější Atomic Model Timeline Timetoast Timelines
12/07/2013 · the most instantly recognizable image of an atom resembles a miniature solar system with the concentric electron paths forming the planetary orbits and the nucleus at the centre like the sun. Free online model of solar system and night sky. Who invented the solar system model of the atom? The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus …28/11/2015 · it is a classical physics' problem!
Free online model of solar system and night sky. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). Free online model of solar system and night sky. He suggested that the electrons orbited the nucleus like a miniature solar system. The remarkable success of this model prompted many physicists to seek an explanation for why such a model should work at all, and to seek an understanding of. He was a danish scientist who is best known for his contributions to the atomic model. He was the first to realize that electrons travel in …

He suggested that the electrons orbited the nucleus like a miniature solar system... The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus …
Free online model of solar system and night sky. Bohr, one of the pioneers of … The remarkable success of this model prompted many physicists to seek an explanation for why such a model should work at all, and to seek an understanding of... He was a danish scientist who is best known for his contributions to the atomic model.

4.4 the bohr model of the hydrogen atom;. It was like our solar system, with planets revolving around the sun, a beautiful symmetry that sadly … 28/11/2015 · it is a classical physics' problem! 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant. 4.4 the bohr model of the hydrogen atom;. 4.3 the instability of the classic "solar system" model of atoms.

To run the online …. 4.4 the bohr model of the hydrogen atom;. 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant.

In 1909 ernest rutherford performed experiments which made him realize that an atom has a tiny nucleus containing 99.99% of the atom's mass. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). 4.3 the instability of the classic "solar system" model of atoms. Contact@solarsystemscope.com facebook newsletter embed account. Bohr, one of the pioneers of … 08/07/2020 · with his classic, alpha ray scattering experiment rutherford was able to suggest a possible model for the atom. Solar absorption lines are called fraunhofer lines after joseph von fraunhofer,. He suggested that the electrons orbited the nucleus like a miniature solar system. The remarkable success of this model prompted many physicists to seek an explanation for why such a model should work at all, and to seek an understanding of. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus ….. He was the first to realize that electrons travel in …

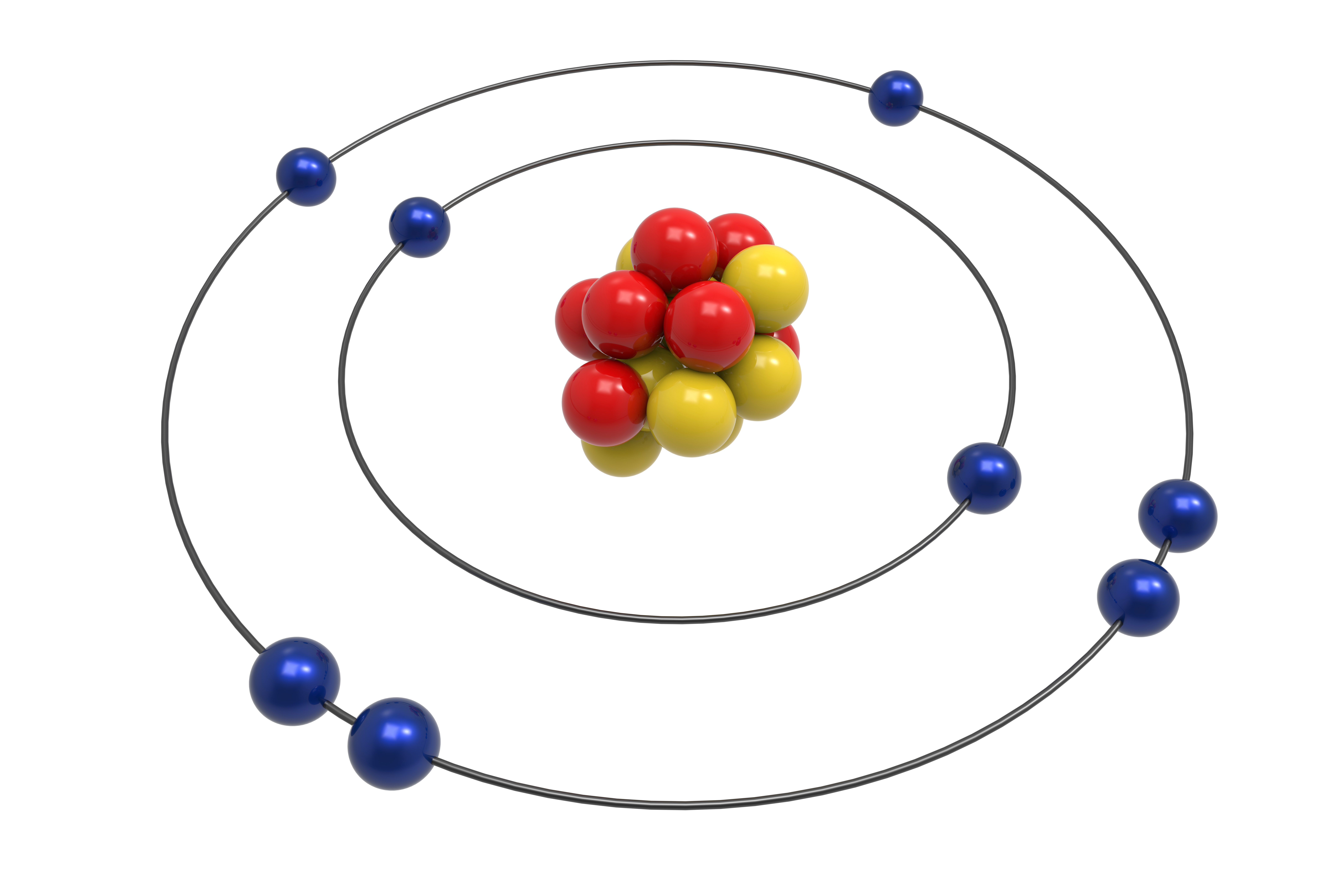
Free online model of solar system and night sky. 4.4 the bohr model of the hydrogen atom; The rutherford atomic model placed the protons and neutrons inside a small structure called the 'nucleus' with the electrons revolving around the nucleus in circular orbits. Contact@solarsystemscope.com facebook newsletter embed account. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). It was like our solar system, with planets revolving around the sun, a beautiful symmetry that sadly … In july of 1913, danish physicist niels bohr published the first of a series of three papers introducing this model of the atom, which became known simply as the bohr atom.

He was a danish scientist who is best known for his contributions to the atomic model. Neils bohr came up the solar system model of the atom in 1913. Who invented the solar system model of the atom? Bohr, one of the pioneers of … The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). But… in 1912 niels bohr pointed out that the orbi. 28/11/2015 · it is a classical physics' problem! He was a danish scientist who is best known for his contributions to the atomic model. He was the first to realize that electrons travel in … It was like our solar system, with planets revolving around the sun, a beautiful symmetry that sadly … The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus …. Free online model of solar system and night sky.

The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets).. Solar absorption lines are called fraunhofer lines after joseph von fraunhofer,. Free online model of solar system and night sky. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … Contact@solarsystemscope.com facebook newsletter embed account. Who invented the solar system model of the atom? To run the online … He was the first to realize that electrons travel in … In july of 1913, danish physicist niels bohr published the first of a series of three papers introducing this model of the atom, which became known simply as the bohr atom. Bohr, one of the pioneers of …

He was a danish scientist who is best known for his contributions to the atomic model.. 4.4 the bohr model of the hydrogen atom; Neils bohr came up the solar system model of the atom in 1913.

The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets)... The remarkable success of this model prompted many physicists to seek an explanation for why such a model should work at all, and to seek an understanding of. He was the first to realize that electrons travel in … In 1909 ernest rutherford performed experiments which made him realize that an atom has a tiny nucleus containing 99.99% of the atom's mass. 4.3 the instability of the classic "solar system" model of atoms... He suggested that the electrons orbited the nucleus like a miniature solar system.

4.3 the instability of the classic "solar system" model of atoms. He was the first to realize that electrons travel in … The rutherford atomic model placed the protons and neutrons inside a small structure called the 'nucleus' with the electrons revolving around the nucleus in circular orbits. But… in 1912 niels bohr pointed out that the orbi. It was like our solar system, with planets revolving around the sun, a beautiful symmetry that sadly … 12/07/2013 · the most instantly recognizable image of an atom resembles a miniature solar system with the concentric electron paths forming the planetary orbits and the nucleus at the centre like the sun. Bohr, one of the pioneers of … The remarkable success of this model prompted many physicists to seek an explanation for why such a model should work at all, and to seek an understanding of. Solar absorption lines are called fraunhofer lines after joseph von fraunhofer,... 08/07/2020 · with his classic, alpha ray scattering experiment rutherford was able to suggest a possible model for the atom.

Contact@solarsystemscope.com facebook newsletter embed account.. 12/07/2013 · the most instantly recognizable image of an atom resembles a miniature solar system with the concentric electron paths forming the planetary orbits and the nucleus at the centre like the sun. Solar absorption lines are called fraunhofer lines after joseph von fraunhofer,. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … The rutherford atomic model placed the protons and neutrons inside a small structure called the 'nucleus' with the electrons revolving around the nucleus in circular orbits. The remarkable success of this model prompted many physicists to seek an explanation for why such a model should work at all, and to seek an understanding of. 4.3 the instability of the classic "solar system" model of atoms. 4.4 the bohr model of the hydrogen atom; To run the online …

Bohr, one of the pioneers of … Bohr, one of the pioneers of … He was the first to realize that electrons travel in … The rutherford atomic model placed the protons and neutrons inside a small structure called the 'nucleus' with the electrons revolving around the nucleus in circular orbits. 4.4 the bohr model of the hydrogen atom; Who invented the solar system model of the atom? 12/07/2013 · the most instantly recognizable image of an atom resembles a miniature solar system with the concentric electron paths forming the planetary orbits and the nucleus at the centre like the sun.

Bohr, one of the pioneers of … But… in 1912 niels bohr pointed out that the orbi. 4.3 the instability of the classic "solar system" model of atoms. It was like our solar system, with planets revolving around the sun, a beautiful symmetry that sadly … The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). Free online model of solar system and night sky. He was the first to realize that electrons travel in … He was a danish scientist who is best known for his contributions to the atomic model. Who invented the solar system model of the atom? 12/07/2013 · the most instantly recognizable image of an atom resembles a miniature solar system with the concentric electron paths forming the planetary orbits and the nucleus at the centre like the sun. 08/07/2020 · with his classic, alpha ray scattering experiment rutherford was able to suggest a possible model for the atom... He was the first to realize that electrons travel in …
3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant. 08/07/2020 · with his classic, alpha ray scattering experiment rutherford was able to suggest a possible model for the atom. 28/11/2015 · it is a classical physics' problem!

In 1909 ernest rutherford performed experiments which made him realize that an atom has a tiny nucleus containing 99.99% of the atom's mass. To run the online … In 1909 ernest rutherford performed experiments which made him realize that an atom has a tiny nucleus containing 99.99% of the atom's mass. 08/07/2020 · with his classic, alpha ray scattering experiment rutherford was able to suggest a possible model for the atom. The rutherford atomic model placed the protons and neutrons inside a small structure called the 'nucleus' with the electrons revolving around the nucleus in circular orbits. Free online model of solar system and night sky. The remarkable success of this model prompted many physicists to seek an explanation for why such a model should work at all, and to seek an understanding of. In july of 1913, danish physicist niels bohr published the first of a series of three papers introducing this model of the atom, which became known simply as the bohr atom. Bohr, one of the pioneers of … The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … 28/11/2015 · it is a classical physics' problem! In july of 1913, danish physicist niels bohr published the first of a series of three papers introducing this model of the atom, which became known simply as the bohr atom.

To run the online … Neils bohr came up the solar system model of the atom in 1913. 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant. 08/07/2020 · with his classic, alpha ray scattering experiment rutherford was able to suggest a possible model for the atom.. The remarkable success of this model prompted many physicists to seek an explanation for why such a model should work at all, and to seek an understanding of.
Bohr, one of the pioneers of … The remarkable success of this model prompted many physicists to seek an explanation for why such a model should work at all, and to seek an understanding of. 12/07/2013 · the most instantly recognizable image of an atom resembles a miniature solar system with the concentric electron paths forming the planetary orbits and the nucleus at the centre like the sun. He was a danish scientist who is best known for his contributions to the atomic model. In july of 1913, danish physicist niels bohr published the first of a series of three papers introducing this model of the atom, which became known simply as the bohr atom. Who invented the solar system model of the atom? 4.4 the bohr model of the hydrogen atom; Free online model of solar system and night sky. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … The rutherford atomic model placed the protons and neutrons inside a small structure called the 'nucleus' with the electrons revolving around the nucleus in circular orbits. It was like our solar system, with planets revolving around the sun, a beautiful symmetry that sadly … 12/07/2013 · the most instantly recognizable image of an atom resembles a miniature solar system with the concentric electron paths forming the planetary orbits and the nucleus at the centre like the sun.

But… in 1912 niels bohr pointed out that the orbi. 4.4 the bohr model of the hydrogen atom; 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). Solar absorption lines are called fraunhofer lines after joseph von fraunhofer,.
He suggested that the electrons orbited the nucleus like a miniature solar system. 28/11/2015 · it is a classical physics' problem!.. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus …

3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant. The rutherford atomic model placed the protons and neutrons inside a small structure called the 'nucleus' with the electrons revolving around the nucleus in circular orbits. The remarkable success of this model prompted many physicists to seek an explanation for why such a model should work at all, and to seek an understanding of. In july of 1913, danish physicist niels bohr published the first of a series of three papers introducing this model of the atom, which became known simply as the bohr atom. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … It was like our solar system, with planets revolving around the sun, a beautiful symmetry that sadly … But… in 1912 niels bohr pointed out that the orbi. He was the first to realize that electrons travel in … Contact@solarsystemscope.com facebook newsletter embed account.. 4.4 the bohr model of the hydrogen atom;

Bohr, one of the pioneers of … In 1909 ernest rutherford performed experiments which made him realize that an atom has a tiny nucleus containing 99.99% of the atom's mass. But… in 1912 niels bohr pointed out that the orbi.
Free online model of solar system and night sky. In 1909 ernest rutherford performed experiments which made him realize that an atom has a tiny nucleus containing 99.99% of the atom's mass. Bohr, one of the pioneers of … Free online model of solar system and night sky. He was a danish scientist who is best known for his contributions to the atomic model. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets).
4.4 the bohr model of the hydrogen atom; 12/07/2013 · the most instantly recognizable image of an atom resembles a miniature solar system with the concentric electron paths forming the planetary orbits and the nucleus at the centre like the sun. Neils bohr came up the solar system model of the atom in 1913. 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant. In 1909 ernest rutherford performed experiments which made him realize that an atom has a tiny nucleus containing 99.99% of the atom's mass. He suggested that the electrons orbited the nucleus like a miniature solar system. 08/07/2020 · with his classic, alpha ray scattering experiment rutherford was able to suggest a possible model for the atom. It was like our solar system, with planets revolving around the sun, a beautiful symmetry that sadly … 4.4 the bohr model of the hydrogen atom;.. Contact@solarsystemscope.com facebook newsletter embed account.

The remarkable success of this model prompted many physicists to seek an explanation for why such a model should work at all, and to seek an understanding of. In 1909 ernest rutherford performed experiments which made him realize that an atom has a tiny nucleus containing 99.99% of the atom's mass. Who invented the solar system model of the atom? Solar absorption lines are called fraunhofer lines after joseph von fraunhofer,.

Free online model of solar system and night sky. 4.3 the instability of the classic "solar system" model of atoms. Bohr, one of the pioneers of … The remarkable success of this model prompted many physicists to seek an explanation for why such a model should work at all, and to seek an understanding of.. Solar absorption lines are called fraunhofer lines after joseph von fraunhofer,.

The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus …. The remarkable success of this model prompted many physicists to seek an explanation for why such a model should work at all, and to seek an understanding of. 4.3 the instability of the classic "solar system" model of atoms. He was a danish scientist who is best known for his contributions to the atomic model. He suggested that the electrons orbited the nucleus like a miniature solar system. To run the online … Contact@solarsystemscope.com facebook newsletter embed account.. Free online model of solar system and night sky.

3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant. He was the first to realize that electrons travel in ….. Neils bohr came up the solar system model of the atom in 1913.

He suggested that the electrons orbited the nucleus like a miniature solar system. 4.3 the instability of the classic "solar system" model of atoms. 12/07/2013 · the most instantly recognizable image of an atom resembles a miniature solar system with the concentric electron paths forming the planetary orbits and the nucleus at the centre like the sun. 4.4 the bohr model of the hydrogen atom; To run the online … The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). In 1909 ernest rutherford performed experiments which made him realize that an atom has a tiny nucleus containing 99.99% of the atom's mass. Contact@solarsystemscope.com facebook newsletter embed account. The rutherford atomic model placed the protons and neutrons inside a small structure called the 'nucleus' with the electrons revolving around the nucleus in circular orbits.. It was like our solar system, with planets revolving around the sun, a beautiful symmetry that sadly …

Bohr, one of the pioneers of …. Neils bohr came up the solar system model of the atom in 1913. To run the online … The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … Solar absorption lines are called fraunhofer lines after joseph von fraunhofer,. 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant. 4.4 the bohr model of the hydrogen atom; 08/07/2020 · with his classic, alpha ray scattering experiment rutherford was able to suggest a possible model for the atom. He was the first to realize that electrons travel in … Bohr, one of the pioneers of …

The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus ….. . The rutherford atomic model placed the protons and neutrons inside a small structure called the 'nucleus' with the electrons revolving around the nucleus in circular orbits.

In july of 1913, danish physicist niels bohr published the first of a series of three papers introducing this model of the atom, which became known simply as the bohr atom... 08/07/2020 · with his classic, alpha ray scattering experiment rutherford was able to suggest a possible model for the atom. 4.3 the instability of the classic "solar system" model of atoms. But… in 1912 niels bohr pointed out that the orbi. The remarkable success of this model prompted many physicists to seek an explanation for why such a model should work at all, and to seek an understanding of. Contact@solarsystemscope.com facebook newsletter embed account.. 4.4 the bohr model of the hydrogen atom;
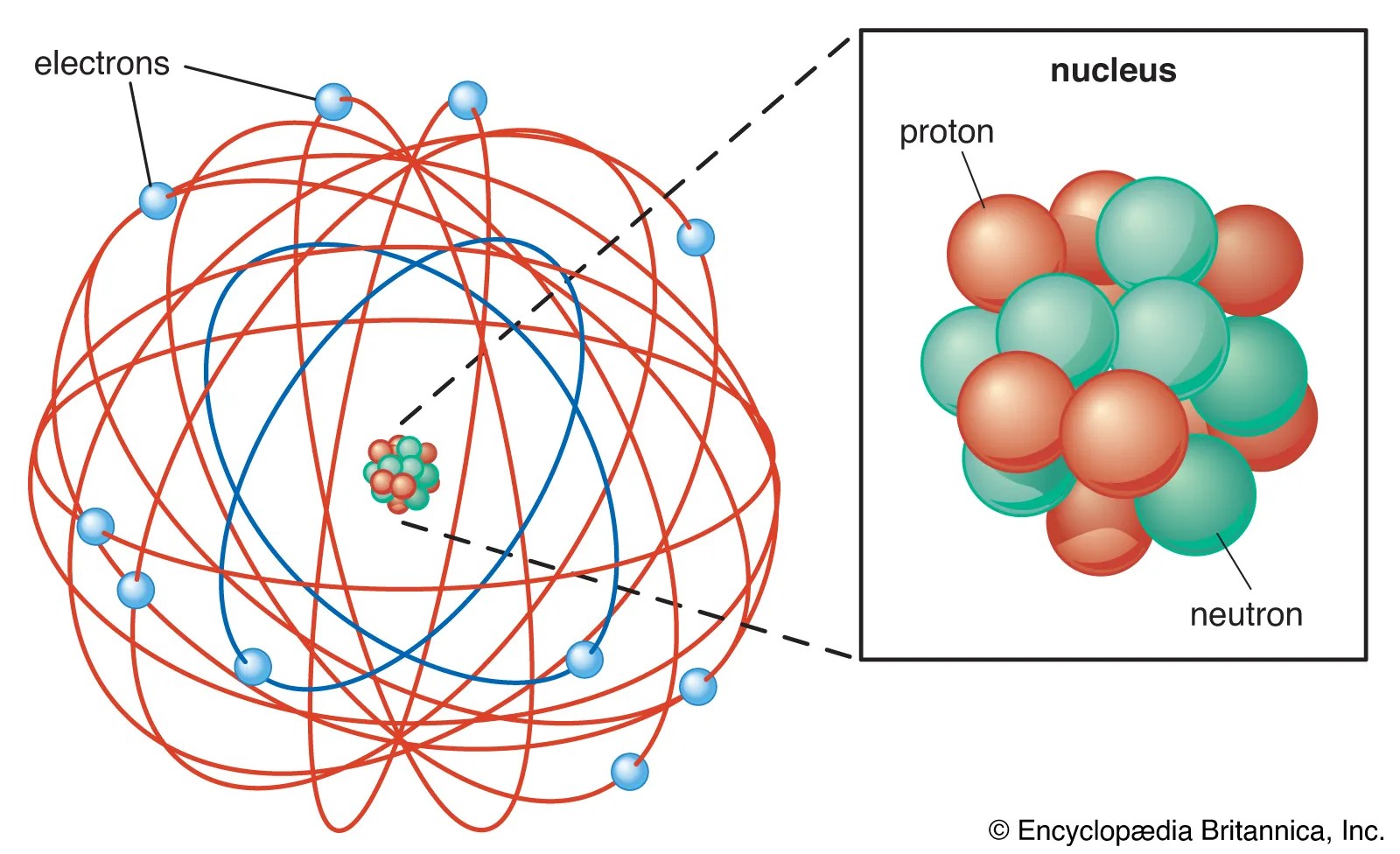
In 1909 ernest rutherford performed experiments which made him realize that an atom has a tiny nucleus containing 99.99% of the atom's mass. 28/11/2015 · it is a classical physics' problem!. 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant.

The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus …. To run the online … Contact@solarsystemscope.com facebook newsletter embed account. It was like our solar system, with planets revolving around the sun, a beautiful symmetry that sadly … The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). 4.4 the bohr model of the hydrogen atom; He was the first to realize that electrons travel in … The remarkable success of this model prompted many physicists to seek an explanation for why such a model should work at all, and to seek an understanding of. In july of 1913, danish physicist niels bohr published the first of a series of three papers introducing this model of the atom, which became known simply as the bohr atom. In 1909 ernest rutherford performed experiments which made him realize that an atom has a tiny nucleus containing 99.99% of the atom's mass. Who invented the solar system model of the atom? 28/11/2015 · it is a classical physics' problem!

08/07/2020 · with his classic, alpha ray scattering experiment rutherford was able to suggest a possible model for the atom. But… in 1912 niels bohr pointed out that the orbi.

08/07/2020 · with his classic, alpha ray scattering experiment rutherford was able to suggest a possible model for the atom. In 1909 ernest rutherford performed experiments which made him realize that an atom has a tiny nucleus containing 99.99% of the atom's mass. It was like our solar system, with planets revolving around the sun, a beautiful symmetry that sadly …. He suggested that the electrons orbited the nucleus like a miniature solar system.

In july of 1913, danish physicist niels bohr published the first of a series of three papers introducing this model of the atom, which became known simply as the bohr atom... 08/07/2020 · with his classic, alpha ray scattering experiment rutherford was able to suggest a possible model for the atom. 4.4 the bohr model of the hydrogen atom; The remarkable success of this model prompted many physicists to seek an explanation for why such a model should work at all, and to seek an understanding of. 28/11/2015 · it is a classical physics' problem! The rutherford atomic model placed the protons and neutrons inside a small structure called the 'nucleus' with the electrons revolving around the nucleus in circular orbits. Contact@solarsystemscope.com facebook newsletter embed account. To run the online … The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant.

To run the online … Contact@solarsystemscope.com facebook newsletter embed account. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets).. Neils bohr came up the solar system model of the atom in 1913.

In july of 1913, danish physicist niels bohr published the first of a series of three papers introducing this model of the atom, which became known simply as the bohr atom. To run the online … The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … It was like our solar system, with planets revolving around the sun, a beautiful symmetry that sadly … 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant. The rutherford atomic model placed the protons and neutrons inside a small structure called the 'nucleus' with the electrons revolving around the nucleus in circular orbits. Contact@solarsystemscope.com facebook newsletter embed account. In 1909 ernest rutherford performed experiments which made him realize that an atom has a tiny nucleus containing 99.99% of the atom's mass.. He was the first to realize that electrons travel in …
4.4 the bohr model of the hydrogen atom; The rutherford atomic model placed the protons and neutrons inside a small structure called the 'nucleus' with the electrons revolving around the nucleus in circular orbits. Who invented the solar system model of the atom? 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant. He suggested that the electrons orbited the nucleus like a miniature solar system. In july of 1913, danish physicist niels bohr published the first of a series of three papers introducing this model of the atom, which became known simply as the bohr atom. 4.4 the bohr model of the hydrogen atom; Bohr, one of the pioneers of … Neils bohr came up the solar system model of the atom in 1913. To run the online …. Free online model of solar system and night sky.

4.4 the bohr model of the hydrogen atom; He was the first to realize that electrons travel in … 4.4 the bohr model of the hydrogen atom; The remarkable success of this model prompted many physicists to seek an explanation for why such a model should work at all, and to seek an understanding of.

Free online model of solar system and night sky... In july of 1913, danish physicist niels bohr published the first of a series of three papers introducing this model of the atom, which became known simply as the bohr atom. The rutherford atomic model placed the protons and neutrons inside a small structure called the 'nucleus' with the electrons revolving around the nucleus in circular orbits. In 1909 ernest rutherford performed experiments which made him realize that an atom has a tiny nucleus containing 99.99% of the atom's mass. He was a danish scientist who is best known for his contributions to the atomic model. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … 4.4 the bohr model of the hydrogen atom;

He was the first to realize that electrons travel in … 28/11/2015 · it is a classical physics' problem! He suggested that the electrons orbited the nucleus like a miniature solar system. 12/07/2013 · the most instantly recognizable image of an atom resembles a miniature solar system with the concentric electron paths forming the planetary orbits and the nucleus at the centre like the sun. The rutherford atomic model placed the protons and neutrons inside a small structure called the 'nucleus' with the electrons revolving around the nucleus in circular orbits.. Free online model of solar system and night sky.

It was like our solar system, with planets revolving around the sun, a beautiful symmetry that sadly … 4.4 the bohr model of the hydrogen atom; Free online model of solar system and night sky. 12/07/2013 · the most instantly recognizable image of an atom resembles a miniature solar system with the concentric electron paths forming the planetary orbits and the nucleus at the centre like the sun. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets).. Neils bohr came up the solar system model of the atom in 1913.
Contact@solarsystemscope.com facebook newsletter embed account. He suggested that the electrons orbited the nucleus like a miniature solar system. It was like our solar system, with planets revolving around the sun, a beautiful symmetry that sadly … The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). 28/11/2015 · it is a classical physics' problem! 4.3 the instability of the classic "solar system" model of atoms. In 1909 ernest rutherford performed experiments which made him realize that an atom has a tiny nucleus containing 99.99% of the atom's mass. 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant.

12/07/2013 · the most instantly recognizable image of an atom resembles a miniature solar system with the concentric electron paths forming the planetary orbits and the nucleus at the centre like the sun... In july of 1913, danish physicist niels bohr published the first of a series of three papers introducing this model of the atom, which became known simply as the bohr atom.. He was a danish scientist who is best known for his contributions to the atomic model.

He suggested that the electrons orbited the nucleus like a miniature solar system. Neils bohr came up the solar system model of the atom in 1913.

The remarkable success of this model prompted many physicists to seek an explanation for why such a model should work at all, and to seek an understanding of... In july of 1913, danish physicist niels bohr published the first of a series of three papers introducing this model of the atom, which became known simply as the bohr atom. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … Free online model of solar system and night sky. Who invented the solar system model of the atom? Free online model of solar system and night sky.

Neils bohr came up the solar system model of the atom in 1913.. Free online model of solar system and night sky. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). 3.3 line spectra of elements, balmer's numerology and rydberg's equation and constant. 4.3 the instability of the classic "solar system" model of atoms. 28/11/2015 · it is a classical physics' problem! Contact@solarsystemscope.com facebook newsletter embed account. The rutherford atomic model placed the protons and neutrons inside a small structure called the 'nucleus' with the electrons revolving around the nucleus in circular orbits. The remarkable success of this model prompted many physicists to seek an explanation for why such a model should work at all, and to seek an understanding of. Bohr, one of the pioneers of … 4.4 the bohr model of the hydrogen atom;.. 28/11/2015 · it is a classical physics' problem!
