70 Atom Nitrogen Vynikající
70 Atom Nitrogen Vynikající. Nitrogen is the seventh element with a total of 7 electrons. So nitrogen gas is very common and plentiful. 2, is a compound made when two nitrogen atoms form a chemical bond. However, only a specialized group of … Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron …
Nejlepší Solved Part 1 Atomic Structure Use Your Knowledge Of Atomic Chegg Com
Therefore the n electron configuration will be 1s In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. That is, the number of electrons and protons in the nitrogen atom is seven.Therefore the n electron configuration will be 1s
Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … The remaining three electrons will go in the 2p orbital. 2, makes up a little less than 20% of the atmosphere. It makes up about 80% of the atmosphere, while oxygen gas, o. After creating a single bond between the atoms, both atoms have 6 electrons each.

Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron …. So nitrogen gas is very common and plentiful. Nitrogen atom, juga dikenal sebagai nitrogen aktif, sangat reaktif, berbentuk triradikal dengan tiga elektron yang tidak berpasangan. 6 electrons to make the correct structure. 2, makes up a little less than 20% of the atmosphere. As per the octet rule, still each atom needs two more electrons to complete its outermost shell... 17.11.2021 · to follow the octet rule (eight electrons per atom), each nitrogen atom needs 3 more electrons i.e.

Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron …. The remaining three electrons will go in the 2p orbital. 17.11.2021 · to follow the octet rule (eight electrons per atom), each nitrogen atom needs 3 more electrons i.e. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Nitrogen is the seventh element with a total of 7 electrons. We can write the formula as, mass of one atom of nitrogen=$\dfrac{{{\text{mass}}\,{\text{of}}\,\,{\text{1}}\,{\text{mole}}}}{{{\text{avogadro's}}\,\,{\text{number}}}}$ However, only a specialized group of … So nitrogen gas is very common and plentiful. Nitrogen is an element that can combine with itself or with other elements to make different compounds. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital.. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital.

For instance nitrogen gas, n.. Nitrogen is an element that can combine with itself or with other elements to make different compounds. After creating a single bond between the atoms, both atoms have 6 electrons each. We can calculate the mass of one atom of nitrogen by dividing the mass of one mole of nitrogen to the avogadro number of atoms. The valency of a nitrogen atom is 3, 5 and the valence electrons of a nitrogen atom are five. Nitrogen is the seventh element with a total of 7 electrons. The active atomic mass of the nitrogen atom is 14.00643, 14.00728. Therefore the n electron configuration will be 1s The active atomic mass of the nitrogen atom is 14.00643, 14.00728.

The remaining three electrons will go in the 2p orbital. The remaining three electrons will go in the 2p orbital. Nitrogen is the seventh element with a total of 7 electrons. Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital. 6 electrons to make the correct structure. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. So nitrogen gas is very common and plentiful... Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron …

That is, the number of electrons and protons in the nitrogen atom is seven.. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … As per the octet rule, still each atom needs two more electrons to complete its outermost shell. The active atomic mass of the nitrogen atom is 14.00643, 14.00728. However, only a specialized group of … So nitrogen gas is very common and plentiful. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital.. It makes up about 80% of the atmosphere, while oxygen gas, o.

We can calculate the mass of one atom of nitrogen by dividing the mass of one mole of nitrogen to the avogadro number of atoms. The valency of a nitrogen atom is 3, 5 and the valence electrons of a nitrogen atom are five. 2, makes up a little less than 20% of the atmosphere. Nitrogen atom, juga dikenal sebagai nitrogen aktif, sangat reaktif, berbentuk triradikal dengan tiga elektron yang tidak berpasangan. Nitrogen is the seventh element with a total of 7 electrons. That is, the number of electrons and protons in the nitrogen atom is seven. 17.11.2021 · to follow the octet rule (eight electrons per atom), each nitrogen atom needs 3 more electrons i.e. Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital.. Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital.

Nitrogen is an element that can combine with itself or with other elements to make different compounds. 6 electrons to make the correct structure. It makes up about 80% of the atmosphere, while oxygen gas, o.

2, makes up a little less than 20% of the atmosphere. Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital. Nitrogen is an element that can combine with itself or with other elements to make different compounds. We can write the formula as, mass of one atom of nitrogen=$\dfrac{{{\text{mass}}\,{\text{of}}\,\,{\text{1}}\,{\text{mole}}}}{{{\text{avogadro's}}\,\,{\text{number}}}}$ The active atomic mass of the nitrogen atom is 14.00643, 14.00728.

After creating a single bond between the atoms, both atoms have 6 electrons each. That is, the number of electrons and protons in the nitrogen atom is seven. The valency of a nitrogen atom is 3, 5 and the valence electrons of a nitrogen atom are five. As per the octet rule, still each atom needs two more electrons to complete its outermost shell. After creating a single bond between the atoms, both atoms have 6 electrons each.. The active atomic mass of the nitrogen atom is 14.00643, 14.00728.

Nitrogen atom, juga dikenal sebagai nitrogen aktif, sangat reaktif, berbentuk triradikal dengan tiga elektron yang tidak berpasangan.. Nitrogen is an element that can combine with itself or with other elements to make different compounds.. We can write the formula as, mass of one atom of nitrogen=$\dfrac{{{\text{mass}}\,{\text{of}}\,\,{\text{1}}\,{\text{mole}}}}{{{\text{avogadro's}}\,\,{\text{number}}}}$

As per the octet rule, still each atom needs two more electrons to complete its outermost shell... 6 electrons to make the correct structure. Nitrogen is the seventh element with a total of 7 electrons. It makes up about 80% of the atmosphere, while oxygen gas, o.

The remaining three electrons will go in the 2p orbital... Nitrogen atom, juga dikenal sebagai nitrogen aktif, sangat reaktif, berbentuk triradikal dengan tiga elektron yang tidak berpasangan. We can calculate the mass of one atom of nitrogen by dividing the mass of one mole of nitrogen to the avogadro number of atoms. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … That is, the number of electrons and protons in the nitrogen atom is seven. 17.11.2021 · to follow the octet rule (eight electrons per atom), each nitrogen atom needs 3 more electrons i.e. As per the octet rule, still each atom needs two more electrons to complete its outermost shell... Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital.

In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital.. The remaining three electrons will go in the 2p orbital. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … 2, makes up a little less than 20% of the atmosphere. However, only a specialized group of … As per the octet rule, still each atom needs two more electrons to complete its outermost shell. 17.11.2021 · to follow the octet rule (eight electrons per atom), each nitrogen atom needs 3 more electrons i.e. We can write the formula as, mass of one atom of nitrogen=$\dfrac{{{\text{mass}}\,{\text{of}}\,\,{\text{1}}\,{\text{mole}}}}{{{\text{avogadro's}}\,\,{\text{number}}}}$

2, is a compound made when two nitrogen atoms form a chemical bond. As per the octet rule, still each atom needs two more electrons to complete its outermost shell. We can calculate the mass of one atom of nitrogen by dividing the mass of one mole of nitrogen to the avogadro number of atoms. That is, the number of electrons and protons in the nitrogen atom is seven. 2, makes up a little less than 20% of the atmosphere. 17.11.2021 · to follow the octet rule (eight electrons per atom), each nitrogen atom needs 3 more electrons i.e. Nitrogen is the seventh element with a total of 7 electrons.. 2, makes up a little less than 20% of the atmosphere.

The remaining three electrons will go in the 2p orbital. For instance nitrogen gas, n. The active atomic mass of the nitrogen atom is 14.00643, 14.00728. 17.11.2021 · to follow the octet rule (eight electrons per atom), each nitrogen atom needs 3 more electrons i.e. However, only a specialized group of … So nitrogen gas is very common and plentiful. That is, the number of electrons and protons in the nitrogen atom is seven. Nitrogen is an element that can combine with itself or with other elements to make different compounds. We can calculate the mass of one atom of nitrogen by dividing the mass of one mole of nitrogen to the avogadro number of atoms. Nitrogen is the seventh element with a total of 7 electrons.
2, is a compound made when two nitrogen atoms form a chemical bond. 2, makes up a little less than 20% of the atmosphere. The valency of a nitrogen atom is 3, 5 and the valence electrons of a nitrogen atom are five. 17.11.2021 · to follow the octet rule (eight electrons per atom), each nitrogen atom needs 3 more electrons i.e. Nitrogen is the seventh element with a total of 7 electrons. It makes up about 80% of the atmosphere, while oxygen gas, o. However, only a specialized group of … Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital.

We can write the formula as, mass of one atom of nitrogen=$\dfrac{{{\text{mass}}\,{\text{of}}\,\,{\text{1}}\,{\text{mole}}}}{{{\text{avogadro's}}\,\,{\text{number}}}}$ As per the octet rule, still each atom needs two more electrons to complete its outermost shell. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital. It makes up about 80% of the atmosphere, while oxygen gas, o. 2, makes up a little less than 20% of the atmosphere.

Therefore the n electron configuration will be 1s. It makes up about 80% of the atmosphere, while oxygen gas, o. As per the octet rule, still each atom needs two more electrons to complete its outermost shell... We can calculate the mass of one atom of nitrogen by dividing the mass of one mole of nitrogen to the avogadro number of atoms.

The valency of a nitrogen atom is 3, 5 and the valence electrons of a nitrogen atom are five... That is, the number of electrons and protons in the nitrogen atom is seven. Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. For instance nitrogen gas, n. Nitrogen atom, juga dikenal sebagai nitrogen aktif, sangat reaktif, berbentuk triradikal dengan tiga elektron yang tidak berpasangan. Therefore the n electron configuration will be 1s Nitrogen is the seventh element with a total of 7 electrons.. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital.
Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital. We can write the formula as, mass of one atom of nitrogen=$\dfrac{{{\text{mass}}\,{\text{of}}\,\,{\text{1}}\,{\text{mole}}}}{{{\text{avogadro's}}\,\,{\text{number}}}}$ Therefore the n electron configuration will be 1s However, only a specialized group of … Nitrogen is an element that can combine with itself or with other elements to make different compounds.. Nitrogen atom, juga dikenal sebagai nitrogen aktif, sangat reaktif, berbentuk triradikal dengan tiga elektron yang tidak berpasangan.

So nitrogen gas is very common and plentiful. For instance nitrogen gas, n. It makes up about 80% of the atmosphere, while oxygen gas, o. Nitrogen atom, juga dikenal sebagai nitrogen aktif, sangat reaktif, berbentuk triradikal dengan tiga elektron yang tidak berpasangan.

17.11.2021 · to follow the octet rule (eight electrons per atom), each nitrogen atom needs 3 more electrons i.e... We can calculate the mass of one atom of nitrogen by dividing the mass of one mole of nitrogen to the avogadro number of atoms. That is, the number of electrons and protons in the nitrogen atom is seven. 6 electrons to make the correct structure. Nitrogen is the seventh element with a total of 7 electrons. Nitrogen is an element that can combine with itself or with other elements to make different compounds. After creating a single bond between the atoms, both atoms have 6 electrons each. The valency of a nitrogen atom is 3, 5 and the valence electrons of a nitrogen atom are five.

Therefore the n electron configuration will be 1s So nitrogen gas is very common and plentiful. However, only a specialized group of … 2, is a compound made when two nitrogen atoms form a chemical bond. We can calculate the mass of one atom of nitrogen by dividing the mass of one mole of nitrogen to the avogadro number of atoms.. Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital.

After creating a single bond between the atoms, both atoms have 6 electrons each. Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital. So nitrogen gas is very common and plentiful. 2, makes up a little less than 20% of the atmosphere. It makes up about 80% of the atmosphere, while oxygen gas, o. 6 electrons to make the correct structure. 17.11.2021 · to follow the octet rule (eight electrons per atom), each nitrogen atom needs 3 more electrons i.e. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron …

2, is a compound made when two nitrogen atoms form a chemical bond. 2, makes up a little less than 20% of the atmosphere. It makes up about 80% of the atmosphere, while oxygen gas, o. The remaining three electrons will go in the 2p orbital. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … For instance nitrogen gas, n. The active atomic mass of the nitrogen atom is 14.00643, 14.00728. As per the octet rule, still each atom needs two more electrons to complete its outermost shell. Nitrogen is the seventh element with a total of 7 electrons... As per the octet rule, still each atom needs two more electrons to complete its outermost shell.

Nitrogen is an element that can combine with itself or with other elements to make different compounds. 2, makes up a little less than 20% of the atmosphere. We can calculate the mass of one atom of nitrogen by dividing the mass of one mole of nitrogen to the avogadro number of atoms. The valency of a nitrogen atom is 3, 5 and the valence electrons of a nitrogen atom are five. 6 electrons to make the correct structure. Nitrogen is the seventh element with a total of 7 electrons. Nitrogen is an element that can combine with itself or with other elements to make different compounds.. So nitrogen gas is very common and plentiful.

Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital.. That is, the number of electrons and protons in the nitrogen atom is seven. However, only a specialized group of … Nitrogen is the seventh element with a total of 7 electrons. 2, makes up a little less than 20% of the atmosphere. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital. For instance nitrogen gas, n. The active atomic mass of the nitrogen atom is 14.00643, 14.00728. We can write the formula as, mass of one atom of nitrogen=$\dfrac{{{\text{mass}}\,{\text{of}}\,\,{\text{1}}\,{\text{mole}}}}{{{\text{avogadro's}}\,\,{\text{number}}}}$. 17.11.2021 · to follow the octet rule (eight electrons per atom), each nitrogen atom needs 3 more electrons i.e.

That is, the number of electrons and protons in the nitrogen atom is seven. The active atomic mass of the nitrogen atom is 14.00643, 14.00728.. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital.

For instance nitrogen gas, n.. 2, makes up a little less than 20% of the atmosphere. The active atomic mass of the nitrogen atom is 14.00643, 14.00728. So nitrogen gas is very common and plentiful. That is, the number of electrons and protons in the nitrogen atom is seven. Therefore the n electron configuration will be 1s 17.11.2021 · to follow the octet rule (eight electrons per atom), each nitrogen atom needs 3 more electrons i.e.. We can write the formula as, mass of one atom of nitrogen=$\dfrac{{{\text{mass}}\,{\text{of}}\,\,{\text{1}}\,{\text{mole}}}}{{{\text{avogadro's}}\,\,{\text{number}}}}$

After creating a single bond between the atoms, both atoms have 6 electrons each. Nitrogen atom, juga dikenal sebagai nitrogen aktif, sangat reaktif, berbentuk triradikal dengan tiga elektron yang tidak berpasangan. 2, is a compound made when two nitrogen atoms form a chemical bond. The active atomic mass of the nitrogen atom is 14.00643, 14.00728. Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Therefore the n electron configuration will be 1s However, only a specialized group of … Nitrogen is an element that can combine with itself or with other elements to make different compounds. As per the octet rule, still each atom needs two more electrons to complete its outermost shell.

The valency of a nitrogen atom is 3, 5 and the valence electrons of a nitrogen atom are five. Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital. We can write the formula as, mass of one atom of nitrogen=$\dfrac{{{\text{mass}}\,{\text{of}}\,\,{\text{1}}\,{\text{mole}}}}{{{\text{avogadro's}}\,\,{\text{number}}}}$ 2, makes up a little less than 20% of the atmosphere. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. 2, is a compound made when two nitrogen atoms form a chemical bond. After creating a single bond between the atoms, both atoms have 6 electrons each. However, only a specialized group of … 17.11.2021 · to follow the octet rule (eight electrons per atom), each nitrogen atom needs 3 more electrons i.e. As per the octet rule, still each atom needs two more electrons to complete its outermost shell. Nitrogen is an element that can combine with itself or with other elements to make different compounds.. Nitrogen is an element that can combine with itself or with other elements to make different compounds.

It makes up about 80% of the atmosphere, while oxygen gas, o. 17.11.2021 · to follow the octet rule (eight electrons per atom), each nitrogen atom needs 3 more electrons i.e. Nitrogen is the seventh element with a total of 7 electrons. We can calculate the mass of one atom of nitrogen by dividing the mass of one mole of nitrogen to the avogadro number of atoms. 2, is a compound made when two nitrogen atoms form a chemical bond. 6 electrons to make the correct structure. The remaining three electrons will go in the 2p orbital. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital. The valency of a nitrogen atom is 3, 5 and the valence electrons of a nitrogen atom are five. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron …

As per the octet rule, still each atom needs two more electrons to complete its outermost shell. For instance nitrogen gas, n. That is, the number of electrons and protons in the nitrogen atom is seven. So nitrogen gas is very common and plentiful. It makes up about 80% of the atmosphere, while oxygen gas, o. After creating a single bond between the atoms, both atoms have 6 electrons each. 6 electrons to make the correct structure. That is, the number of electrons and protons in the nitrogen atom is seven.

The valency of a nitrogen atom is 3, 5 and the valence electrons of a nitrogen atom are five.. However, only a specialized group of …. For instance nitrogen gas, n.

6 electrons to make the correct structure.. That is, the number of electrons and protons in the nitrogen atom is seven. The active atomic mass of the nitrogen atom is 14.00643, 14.00728. As per the octet rule, still each atom needs two more electrons to complete its outermost shell. We can calculate the mass of one atom of nitrogen by dividing the mass of one mole of nitrogen to the avogadro number of atoms. The valency of a nitrogen atom is 3, 5 and the valence electrons of a nitrogen atom are five... The active atomic mass of the nitrogen atom is 14.00643, 14.00728.

We can write the formula as, mass of one atom of nitrogen=$\dfrac{{{\text{mass}}\,{\text{of}}\,\,{\text{1}}\,{\text{mole}}}}{{{\text{avogadro's}}\,\,{\text{number}}}}$ 6 electrons to make the correct structure.. After creating a single bond between the atoms, both atoms have 6 electrons each.

It makes up about 80% of the atmosphere, while oxygen gas, o. After creating a single bond between the atoms, both atoms have 6 electrons each. Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. That is, the number of electrons and protons in the nitrogen atom is seven. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … The remaining three electrons will go in the 2p orbital. 17.11.2021 · to follow the octet rule (eight electrons per atom), each nitrogen atom needs 3 more electrons i.e. For instance nitrogen gas, n.

Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … It makes up about 80% of the atmosphere, while oxygen gas, o. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. As per the octet rule, still each atom needs two more electrons to complete its outermost shell. Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital... We can calculate the mass of one atom of nitrogen by dividing the mass of one mole of nitrogen to the avogadro number of atoms.

The remaining three electrons will go in the 2p orbital. As per the octet rule, still each atom needs two more electrons to complete its outermost shell. The valency of a nitrogen atom is 3, 5 and the valence electrons of a nitrogen atom are five. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. 6 electrons to make the correct structure. Nitrogen is the seventh element with a total of 7 electrons.
Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital. 17.11.2021 · to follow the octet rule (eight electrons per atom), each nitrogen atom needs 3 more electrons i.e. It makes up about 80% of the atmosphere, while oxygen gas, o. That is, the number of electrons and protons in the nitrogen atom is seven. As per the octet rule, still each atom needs two more electrons to complete its outermost shell. However, only a specialized group of …

We can write the formula as, mass of one atom of nitrogen=$\dfrac{{{\text{mass}}\,{\text{of}}\,\,{\text{1}}\,{\text{mole}}}}{{{\text{avogadro's}}\,\,{\text{number}}}}$. 6 electrons to make the correct structure. For instance nitrogen gas, n.. For instance nitrogen gas, n.

2, makes up a little less than 20% of the atmosphere... After creating a single bond between the atoms, both atoms have 6 electrons each. Nitrogen is an element that can combine with itself or with other elements to make different compounds. However, only a specialized group of … The remaining three electrons will go in the 2p orbital. We can calculate the mass of one atom of nitrogen by dividing the mass of one mole of nitrogen to the avogadro number of atoms. The active atomic mass of the nitrogen atom is 14.00643, 14.00728. Nitrogen is an element that can combine with itself or with other elements to make different compounds.

In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Therefore the n electron configuration will be 1s. As per the octet rule, still each atom needs two more electrons to complete its outermost shell.
Nitrogen is the seventh element with a total of 7 electrons. .. That is, the number of electrons and protons in the nitrogen atom is seven.

6 electrons to make the correct structure... Nitrogen is an element that can combine with itself or with other elements to make different compounds. So nitrogen gas is very common and plentiful. 6 electrons to make the correct structure. After creating a single bond between the atoms, both atoms have 6 electrons each.

The valency of a nitrogen atom is 3, 5 and the valence electrons of a nitrogen atom are five. As per the octet rule, still each atom needs two more electrons to complete its outermost shell.. After creating a single bond between the atoms, both atoms have 6 electrons each.

Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital. Nitrogen is the seventh element with a total of 7 electrons. Nitrogen is an element that can combine with itself or with other elements to make different compounds. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … We can calculate the mass of one atom of nitrogen by dividing the mass of one mole of nitrogen to the avogadro number of atoms. For instance nitrogen gas, n. After creating a single bond between the atoms, both atoms have 6 electrons each. We can write the formula as, mass of one atom of nitrogen=$\dfrac{{{\text{mass}}\,{\text{of}}\,\,{\text{1}}\,{\text{mole}}}}{{{\text{avogadro's}}\,\,{\text{number}}}}$ So nitrogen gas is very common and plentiful.

So nitrogen gas is very common and plentiful.. Nitrogen atom, juga dikenal sebagai nitrogen aktif, sangat reaktif, berbentuk triradikal dengan tiga elektron yang tidak berpasangan. Nitrogen is the seventh element with a total of 7 electrons.. For instance nitrogen gas, n.

However, only a specialized group of … 2, is a compound made when two nitrogen atoms form a chemical bond. Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. The remaining three electrons will go in the 2p orbital.

It makes up about 80% of the atmosphere, while oxygen gas, o. That is, the number of electrons and protons in the nitrogen atom is seven. The active atomic mass of the nitrogen atom is 14.00643, 14.00728. The remaining three electrons will go in the 2p orbital. We can write the formula as, mass of one atom of nitrogen=$\dfrac{{{\text{mass}}\,{\text{of}}\,\,{\text{1}}\,{\text{mole}}}}{{{\text{avogadro's}}\,\,{\text{number}}}}$ Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital. 2, makes up a little less than 20% of the atmosphere. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron ….. 2, makes up a little less than 20% of the atmosphere.

17.11.2021 · to follow the octet rule (eight electrons per atom), each nitrogen atom needs 3 more electrons i.e. After creating a single bond between the atoms, both atoms have 6 electrons each.. Nitrogen is an element that can combine with itself or with other elements to make different compounds.

The active atomic mass of the nitrogen atom is 14.00643, 14.00728. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … Nitrogen atom, juga dikenal sebagai nitrogen aktif, sangat reaktif, berbentuk triradikal dengan tiga elektron yang tidak berpasangan. Therefore the n electron configuration will be 1s
6 electrons to make the correct structure. 2, makes up a little less than 20% of the atmosphere. Therefore the n electron configuration will be 1s Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital... After creating a single bond between the atoms, both atoms have 6 electrons each.

So nitrogen gas is very common and plentiful... The remaining three electrons will go in the 2p orbital. That is, the number of electrons and protons in the nitrogen atom is seven. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. The active atomic mass of the nitrogen atom is 14.00643, 14.00728. For instance nitrogen gas, n... The remaining three electrons will go in the 2p orbital.

So nitrogen gas is very common and plentiful. Nitrogen is the seventh element with a total of 7 electrons. Nitrogen is an element that can combine with itself or with other elements to make different compounds. However, only a specialized group of …
So nitrogen gas is very common and plentiful. 6 electrons to make the correct structure. We can write the formula as, mass of one atom of nitrogen=$\dfrac{{{\text{mass}}\,{\text{of}}\,\,{\text{1}}\,{\text{mole}}}}{{{\text{avogadro's}}\,\,{\text{number}}}}$ It makes up about 80% of the atmosphere, while oxygen gas, o. Nitrogen is the seventh element with a total of 7 electrons. 2, makes up a little less than 20% of the atmosphere. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. The valency of a nitrogen atom is 3, 5 and the valence electrons of a nitrogen atom are five... 2, makes up a little less than 20% of the atmosphere.
The active atomic mass of the nitrogen atom is 14.00643, 14.00728. We can calculate the mass of one atom of nitrogen by dividing the mass of one mole of nitrogen to the avogadro number of atoms. 2, makes up a little less than 20% of the atmosphere. Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital. It makes up about 80% of the atmosphere, while oxygen gas, o. We can write the formula as, mass of one atom of nitrogen=$\dfrac{{{\text{mass}}\,{\text{of}}\,\,{\text{1}}\,{\text{mole}}}}{{{\text{avogadro's}}\,\,{\text{number}}}}$. After creating a single bond between the atoms, both atoms have 6 electrons each.

We can calculate the mass of one atom of nitrogen by dividing the mass of one mole of nitrogen to the avogadro number of atoms. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … The valency of a nitrogen atom is 3, 5 and the valence electrons of a nitrogen atom are five. Nitrogen atom, juga dikenal sebagai nitrogen aktif, sangat reaktif, berbentuk triradikal dengan tiga elektron yang tidak berpasangan. After creating a single bond between the atoms, both atoms have 6 electrons each. 17.11.2021 · to follow the octet rule (eight electrons per atom), each nitrogen atom needs 3 more electrons i.e. 6 electrons to make the correct structure. That is, the number of electrons and protons in the nitrogen atom is seven. Therefore the n electron configuration will be 1s 17.11.2021 · to follow the octet rule (eight electrons per atom), each nitrogen atom needs 3 more electrons i.e.

Nitrogen is the seventh element with a total of 7 electrons... . Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital.

It makes up about 80% of the atmosphere, while oxygen gas, o. Nitrogen is an element that can combine with itself or with other elements to make different compounds. The remaining three electrons will go in the 2p orbital. As per the octet rule, still each atom needs two more electrons to complete its outermost shell. Nitrogen atom, juga dikenal sebagai nitrogen aktif, sangat reaktif, berbentuk triradikal dengan tiga elektron yang tidak berpasangan. After creating a single bond between the atoms, both atoms have 6 electrons each. The active atomic mass of the nitrogen atom is 14.00643, 14.00728.. 2, makes up a little less than 20% of the atmosphere.

Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital. Nitrogen atom, juga dikenal sebagai nitrogen aktif, sangat reaktif, berbentuk triradikal dengan tiga elektron yang tidak berpasangan. The valency of a nitrogen atom is 3, 5 and the valence electrons of a nitrogen atom are five. The valency of a nitrogen atom is 3, 5 and the valence electrons of a nitrogen atom are five.

We can calculate the mass of one atom of nitrogen by dividing the mass of one mole of nitrogen to the avogadro number of atoms. So nitrogen gas is very common and plentiful. As per the octet rule, still each atom needs two more electrons to complete its outermost shell. After creating a single bond between the atoms, both atoms have 6 electrons each... Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital.

As per the octet rule, still each atom needs two more electrons to complete its outermost shell... 17.11.2021 · to follow the octet rule (eight electrons per atom), each nitrogen atom needs 3 more electrons i.e. 6 electrons to make the correct structure. Therefore the n electron configuration will be 1s It makes up about 80% of the atmosphere, while oxygen gas, o. The remaining three electrons will go in the 2p orbital. For instance nitrogen gas, n. The valency of a nitrogen atom is 3, 5 and the valence electrons of a nitrogen atom are five.

Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital... 2, is a compound made when two nitrogen atoms form a chemical bond. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron …

It makes up about 80% of the atmosphere, while oxygen gas, o.. The remaining three electrons will go in the 2p orbital. Nitrogen atom, juga dikenal sebagai nitrogen aktif, sangat reaktif, berbentuk triradikal dengan tiga elektron yang tidak berpasangan. We can write the formula as, mass of one atom of nitrogen=$\dfrac{{{\text{mass}}\,{\text{of}}\,\,{\text{1}}\,{\text{mole}}}}{{{\text{avogadro's}}\,\,{\text{number}}}}$ Nitrogen is the seventh element with a total of 7 electrons. The active atomic mass of the nitrogen atom is 14.00643, 14.00728. 17.11.2021 · to follow the octet rule (eight electrons per atom), each nitrogen atom needs 3 more electrons i.e. Nitrogen is an element that can combine with itself or with other elements to make different compounds. 2, makes up a little less than 20% of the atmosphere. After creating a single bond between the atoms, both atoms have 6 electrons each. It makes up about 80% of the atmosphere, while oxygen gas, o.

For instance nitrogen gas, n... 2, is a compound made when two nitrogen atoms form a chemical bond. The active atomic mass of the nitrogen atom is 14.00643, 14.00728. Therefore the n electron configuration will be 1s Nitrogen atom, juga dikenal sebagai nitrogen aktif, sangat reaktif, berbentuk triradikal dengan tiga elektron yang tidak berpasangan. The remaining three electrons will go in the 2p orbital. The valency of a nitrogen atom is 3, 5 and the valence electrons of a nitrogen atom are five. However, only a specialized group of … In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. So nitrogen gas is very common and plentiful. 17.11.2021 · to follow the octet rule (eight electrons per atom), each nitrogen atom needs 3 more electrons i.e... Nitrogen is the seventh element with a total of 7 electrons.

17.11.2021 · to follow the octet rule (eight electrons per atom), each nitrogen atom needs 3 more electrons i.e. It makes up about 80% of the atmosphere, while oxygen gas, o. Nitrogen is an element that can combine with itself or with other elements to make different compounds. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. We can calculate the mass of one atom of nitrogen by dividing the mass of one mole of nitrogen to the avogadro number of atoms. Therefore the n electron configuration will be 1s As per the octet rule, still each atom needs two more electrons to complete its outermost shell. Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital. Nitrogen is the seventh element with a total of 7 electrons.. For instance nitrogen gas, n.

17.11.2021 · to follow the octet rule (eight electrons per atom), each nitrogen atom needs 3 more electrons i.e. That is, the number of electrons and protons in the nitrogen atom is seven.
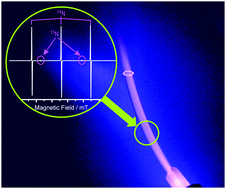
As per the octet rule, still each atom needs two more electrons to complete its outermost shell... Nitrogen is the seventh element with a total of 7 electrons. Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital. 6 electrons to make the correct structure.. We can write the formula as, mass of one atom of nitrogen=$\dfrac{{{\text{mass}}\,{\text{of}}\,\,{\text{1}}\,{\text{mole}}}}{{{\text{avogadro's}}\,\,{\text{number}}}}$

Nitrogen atom, juga dikenal sebagai nitrogen aktif, sangat reaktif, berbentuk triradikal dengan tiga elektron yang tidak berpasangan. Nitrogen is an element that can combine with itself or with other elements to make different compounds. For instance nitrogen gas, n. Nitrogen atom, juga dikenal sebagai nitrogen aktif, sangat reaktif, berbentuk triradikal dengan tiga elektron yang tidak berpasangan. 17.11.2021 · to follow the octet rule (eight electrons per atom), each nitrogen atom needs 3 more electrons i.e.. The valency of a nitrogen atom is 3, 5 and the valence electrons of a nitrogen atom are five.

For instance nitrogen gas, n. The remaining three electrons will go in the 2p orbital. So nitrogen gas is very common and plentiful. 17.11.2021 · to follow the octet rule (eight electrons per atom), each nitrogen atom needs 3 more electrons i.e. 2, is a compound made when two nitrogen atoms form a chemical bond. It makes up about 80% of the atmosphere, while oxygen gas, o. Nitrogen is an element that can combine with itself or with other elements to make different compounds.. 2, makes up a little less than 20% of the atmosphere.

After creating a single bond between the atoms, both atoms have 6 electrons each. Nitrogen is the seventh element with a total of 7 electrons. 2, makes up a little less than 20% of the atmosphere. The valency of a nitrogen atom is 3, 5 and the valence electrons of a nitrogen atom are five. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … The active atomic mass of the nitrogen atom is 14.00643, 14.00728.. That is, the number of electrons and protons in the nitrogen atom is seven.

As per the octet rule, still each atom needs two more electrons to complete its outermost shell. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … Therefore the n electron configuration will be 1s Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital. 2, is a compound made when two nitrogen atoms form a chemical bond. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. As per the octet rule, still each atom needs two more electrons to complete its outermost shell. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital.

After creating a single bond between the atoms, both atoms have 6 electrons each. Nitrogen is an element that can combine with itself or with other elements to make different compounds. We can calculate the mass of one atom of nitrogen by dividing the mass of one mole of nitrogen to the avogadro number of atoms. We can calculate the mass of one atom of nitrogen by dividing the mass of one mole of nitrogen to the avogadro number of atoms.

Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron ….. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. The active atomic mass of the nitrogen atom is 14.00643, 14.00728. Nitrogen is an element that can combine with itself or with other elements to make different compounds. 6 electrons to make the correct structure. After creating a single bond between the atoms, both atoms have 6 electrons each.. Nitrogen is an element that can combine with itself or with other elements to make different compounds.

The remaining three electrons will go in the 2p orbital. After creating a single bond between the atoms, both atoms have 6 electrons each. However, only a specialized group of … 2, makes up a little less than 20% of the atmosphere. Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital.

2, is a compound made when two nitrogen atoms form a chemical bond... Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital. For instance nitrogen gas, n. We can calculate the mass of one atom of nitrogen by dividing the mass of one mole of nitrogen to the avogadro number of atoms. 2, makes up a little less than 20% of the atmosphere. That is, the number of electrons and protons in the nitrogen atom is seven. 2, is a compound made when two nitrogen atoms form a chemical bond. So nitrogen gas is very common and plentiful.. Nitrogen is the seventh element with a total of 7 electrons.

Nitrogen is an element that can combine with itself or with other elements to make different compounds. However, only a specialized group of … The valency of a nitrogen atom is 3, 5 and the valence electrons of a nitrogen atom are five. So nitrogen gas is very common and plentiful. The remaining three electrons will go in the 2p orbital. We can write the formula as, mass of one atom of nitrogen=$\dfrac{{{\text{mass}}\,{\text{of}}\,\,{\text{1}}\,{\text{mole}}}}{{{\text{avogadro's}}\,\,{\text{number}}}}$ 2, makes up a little less than 20% of the atmosphere. As per the octet rule, still each atom needs two more electrons to complete its outermost shell. Nitrogen is an element that can combine with itself or with other elements to make different compounds. It makes up about 80% of the atmosphere, while oxygen gas, o. Therefore the n electron configuration will be 1s. We can write the formula as, mass of one atom of nitrogen=$\dfrac{{{\text{mass}}\,{\text{of}}\,\,{\text{1}}\,{\text{mole}}}}{{{\text{avogadro's}}\,\,{\text{number}}}}$
Nitrogen is the seventh element with a total of 7 electrons. We can calculate the mass of one atom of nitrogen by dividing the mass of one mole of nitrogen to the avogadro number of atoms. The valency of a nitrogen atom is 3, 5 and the valence electrons of a nitrogen atom are five. The active atomic mass of the nitrogen atom is 14.00643, 14.00728. After creating a single bond between the atoms, both atoms have 6 electrons each. So nitrogen gas is very common and plentiful. As per the octet rule, still each atom needs two more electrons to complete its outermost shell. We can write the formula as, mass of one atom of nitrogen=$\dfrac{{{\text{mass}}\,{\text{of}}\,\,{\text{1}}\,{\text{mole}}}}{{{\text{avogadro's}}\,\,{\text{number}}}}$ 2, is a compound made when two nitrogen atoms form a chemical bond.. 2, makes up a little less than 20% of the atmosphere.

The valency of a nitrogen atom is 3, 5 and the valence electrons of a nitrogen atom are five.. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron …. We can calculate the mass of one atom of nitrogen by dividing the mass of one mole of nitrogen to the avogadro number of atoms.

Nitrogen is the seventh element with a total of 7 electrons. Changes in the molecular environment, in interactions or in molecular topology influence nitrogen's electron … 2, is a compound made when two nitrogen atoms form a chemical bond. Nitrogen atom, juga dikenal sebagai nitrogen aktif, sangat reaktif, berbentuk triradikal dengan tiga elektron yang tidak berpasangan. 2, makes up a little less than 20% of the atmosphere. As per the octet rule, still each atom needs two more electrons to complete its outermost shell. Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital. However, only a specialized group of …