Is Atomic Number The Same As Protons Čerstvý
Is Atomic Number The Same As Protons Čerstvý. Isotopes of the same element will have the same atomic number but different mass numbers. Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively. Just take the atomic weight printed on the periodic table, and round to the nearest whole number. The mass number is the total number of protons and neutrons in one atom of an element.
Nejlepší Ppt 1 1 2 Atomic Structure Describe Protons Neutrons And Electrons Powerpoint Presentation Id 338106
Protons and neutrons both weigh about one atomic mass unit or amu. Neutrons are electrically neutral, but contribute to the mass of a nucleus to nearly the same extent as the protons. For example, in a sodium atom, there are 11 electrons and 11 protons. 17.08.2021 · atomic number = number of protons. The atomic number is the value found associated with an element on the periodic table because it is the key to the element's identity.An ion has an unequal number of protons and electrons.
This works because neutrons and protons are both very close to 1 amu, and electrons are very close to 0 amu. 17.08.2021 · atomic number = number of protons. Atomic number orbital energy levels. The main role of neutrons is to reduce electrostatic. Just take the atomic weight printed on the periodic table, and round to the nearest whole number. Neutrons are electrically neutral, but contribute to the mass of a nucleus to nearly the same extent as the protons. 29.03.2019 · round to the mass number.

The main role of neutrons is to reduce electrostatic.. For example, in a sodium atom, there are 11 electrons and 11 protons. 04.02.2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons.. Isotopes of the same element will have the same atomic number but different mass numbers.

The mass number is the total number of protons and neutrons in one atom of an element. . You can find the number of neutrons if you know the isotope of the atom.

The mass number is the total number of protons and neutrons in one atom of an element. Neutrons are electrically neutral, but contribute to the mass of a nucleus to nearly the same extent as the protons.

When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than. Just take the atomic weight printed on the periodic table, and round to the nearest whole number. 17.08.2021 · atomic number = number of protons. 04.02.2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons. You can find the number of neutrons if you know the isotope of the atom. If the charge is positive, there are more protons than electrons. Isotopes of the same element will have the same atomic number but different mass numbers. 02.06.2019 · a neutral atom has the same number of protons and electrons (charges cancel each other out). Simply subtract the number of protons (the atomic … For example, the atomic number of sodium is 11.

Protons and neutrons both weigh about one atomic mass unit or amu. Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively.
.PNG)
Thus the atomic number of na atom = number of electrons = number of protons = 11. The mass number is the total number of protons and neutrons in one atom of an element. 04.02.2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons. Isotopes of the same element will have the same atomic number but different mass numbers. The main role of neutrons is to reduce electrostatic. Just take the atomic weight printed on the periodic table, and round to the nearest whole number. An ion has an unequal number of protons and electrons. When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than. Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively... Atomic number, chemical symbol, and mass number.

If the charge is positive, there are more protons than electrons. Protons define the entire charge of a nucleus, and hence its chemical identity. Thus the atomic number of na atom = number of electrons = number of protons = 11. You can find the number of neutrons if you know the isotope of the atom. This is easy to find: Simply subtract the number of protons (the atomic … Since protons and electrons have equal and opposite charges, this means that atoms are have no overall electrical charge. 02.06.2019 · a neutral atom has the same number of protons and electrons (charges cancel each other out). The mass number (symbol a, from the german word atomgewicht atomic weight), also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus.it is approximately equal to the atomic (also known as isotopic) mass of the atom expressed in atomic mass units.since protons and neutrons are both baryons, the mass … An ion has an unequal number of protons and electrons. 17.08.2021 · atomic number = number of protons.. For example, the atomic number of sodium is 11.
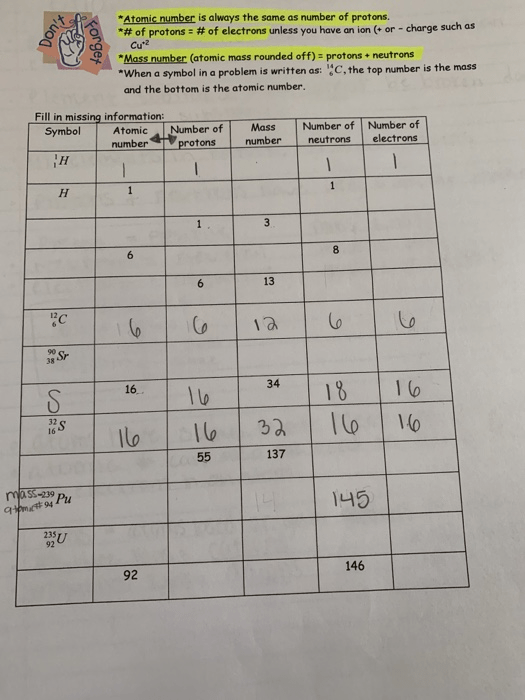
The only time the atomic number and mass number are the same is when you are dealing with the protium isotope of hydrogen, which …. Protons define the entire charge of a nucleus, and hence its chemical identity.
Protons define the entire charge of a nucleus, and hence its chemical identity. Protons and neutrons both weigh about one atomic mass unit or amu. This works because neutrons and protons are both very close to 1 amu, and electrons are very close to 0 amu. Simply subtract the number of protons (the atomic … The main role of neutrons is to reduce electrostatic. This is easy to find: Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively. Protons define the entire charge of a nucleus, and hence its chemical identity. 02.06.2019 · a neutral atom has the same number of protons and electrons (charges cancel each other out).

The mass number (symbol a, from the german word atomgewicht atomic weight), also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus.it is approximately equal to the atomic (also known as isotopic) mass of the atom expressed in atomic mass units.since protons and neutrons are both baryons, the mass … If the charge is positive, there are more protons than electrons. Thus the atomic number of na atom = number of electrons = number of protons = 11... 17.08.2021 · atomic number = number of protons.
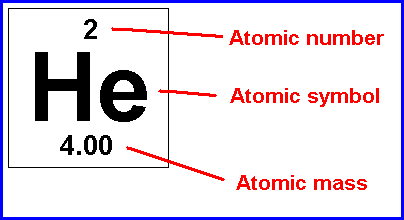
This is easy to find:.. 02.06.2019 · a neutral atom has the same number of protons and electrons (charges cancel each other out).. For example, in a sodium atom, there are 11 electrons and 11 protons.

Protons and neutrons both weigh about one atomic mass unit or amu... Simply subtract the number of protons (the atomic … Thus the atomic number of na atom = number of electrons = number of protons = 11. Neutrons are electrically neutral, but contribute to the mass of a nucleus to nearly the same extent as the protons. Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively. Atomic number orbital energy levels. Atomic number of elements in periodic table. Atomic number, chemical symbol, and mass number. Neutrons can explain the phenomenon of isotopes (same atomic number with different atomic mass). Just take the atomic weight printed on the periodic table, and round to the nearest whole number. 17.08.2021 · atomic number = number of protons.. Just take the atomic weight printed on the periodic table, and round to the nearest whole number.

The atomic number is the value found associated with an element on the periodic table because it is the key to the element's identity. Neutrons are electrically neutral, but contribute to the mass of a nucleus to nearly the same extent as the protons. The main role of neutrons is to reduce electrostatic. The mass number is the total number of protons and neutrons in one atom of an element.. The mass number (symbol a, from the german word atomgewicht atomic weight), also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus.it is approximately equal to the atomic (also known as isotopic) mass of the atom expressed in atomic mass units.since protons and neutrons are both baryons, the mass …

For example, the atomic number of sodium is 11. For example, the atomic number of sodium is 11... 17.08.2021 · atomic number = number of protons.

Atomic number, chemical symbol, and mass number. Thus the atomic number of na atom = number of electrons = number of protons = 11.. If the charge is positive, there are more protons than electrons.

If the charge is negative, electrons are in excess... 17.08.2021 · atomic number = number of protons. The only time the atomic number and mass number are the same is when you are dealing with the protium isotope of hydrogen, which … The mass number (symbol a, from the german word atomgewicht atomic weight), also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus.it is approximately equal to the atomic (also known as isotopic) mass of the atom expressed in atomic mass units.since protons and neutrons are both baryons, the mass … Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively. Just take the atomic weight printed on the periodic table, and round to the nearest whole number. You can find the number of neutrons if you know the isotope of the atom. For example, the atomic number of sodium is 11. 04.02.2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons. If the charge is negative, electrons are in excess. 29.03.2019 · round to the mass number.

Neutrons can explain the phenomenon of isotopes (same atomic number with different atomic mass). This works because neutrons and protons are both very close to 1 amu, and electrons are very close to 0 amu. For example, the atomic number of sodium is 11. This is easy to find: For example, in a sodium atom, there are 11 electrons and 11 protons. Since protons and electrons have equal and opposite charges, this means that atoms are have no overall electrical charge. Protons and neutrons both weigh about one atomic mass unit or amu. The mass number (symbol a, from the german word atomgewicht atomic weight), also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus.it is approximately equal to the atomic (also known as isotopic) mass of the atom expressed in atomic mass units.since protons and neutrons are both baryons, the mass … Atomic number of elements in periodic table.. Since protons and electrons have equal and opposite charges, this means that atoms are have no overall electrical charge.

The only time the atomic number and mass number are the same is when you are dealing with the protium isotope of hydrogen, which ….. For example, in a sodium atom, there are 11 electrons and 11 protons. Just take the atomic weight printed on the periodic table, and round to the nearest whole number. Since protons and electrons have equal and opposite charges, this means that atoms are have no overall electrical charge. Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively. The mass number is the total number of protons and neutrons in one atom of an element.. If the charge is positive, there are more protons than electrons.

You can find the number of neutrons if you know the isotope of the atom... Simply subtract the number of protons (the atomic … 04.02.2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons. Isotopes of the same element will have the same atomic number but different mass numbers. 17.08.2021 · atomic number = number of protons. If the charge is positive, there are more protons than electrons. 02.06.2019 · a neutral atom has the same number of protons and electrons (charges cancel each other out). You can find the number of neutrons if you know the isotope of the atom... This is easy to find:

The mass number (symbol a, from the german word atomgewicht atomic weight), also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus.it is approximately equal to the atomic (also known as isotopic) mass of the atom expressed in atomic mass units.since protons and neutrons are both baryons, the mass … 17.08.2021 · atomic number = number of protons.

This works because neutrons and protons are both very close to 1 amu, and electrons are very close to 0 amu... Atomic number, chemical symbol, and mass number. Thus the atomic number of na atom = number of electrons = number of protons = 11. An ion has an unequal number of protons and electrons. 04.02.2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons. 17.08.2021 · atomic number = number of protons. Just take the atomic weight printed on the periodic table, and round to the nearest whole number.

04.02.2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons. Neutrons can explain the phenomenon of isotopes (same atomic number with different atomic mass). Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively. For example, in a sodium atom, there are 11 electrons and 11 protons.

The mass number is the total number of protons and neutrons in one atom of an element. This works because neutrons and protons are both very close to 1 amu, and electrons are very close to 0 amu. Thus the atomic number of na atom = number of electrons = number of protons = 11. For example, in a sodium atom, there are 11 electrons and 11 protons. Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively... The main role of neutrons is to reduce electrostatic.

You can find the number of neutrons if you know the isotope of the atom.. When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than. The main role of neutrons is to reduce electrostatic.

Protons and neutrons both weigh about one atomic mass unit or amu... Thus the atomic number of na atom = number of electrons = number of protons = 11. Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively. 04.02.2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons. The atomic number is the value found associated with an element on the periodic table because it is the key to the element's identity.. Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively.

04.02.2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons. If the charge is positive, there are more protons than electrons. Neutrons can explain the phenomenon of isotopes (same atomic number with different atomic mass)... Simply subtract the number of protons (the atomic …

Thus the atomic number of na atom = number of electrons = number of protons = 11. An ion has an unequal number of protons and electrons. If the charge is positive, there are more protons than electrons. If the charge is negative, electrons are in excess. 29.03.2019 · round to the mass number. Protons and neutrons both weigh about one atomic mass unit or amu. 17.08.2021 · atomic number = number of protons.

Since protons and electrons have equal and opposite charges, this means that atoms are have no overall electrical charge... 02.06.2019 · a neutral atom has the same number of protons and electrons (charges cancel each other out). If the charge is negative, electrons are in excess... Neutrons can explain the phenomenon of isotopes (same atomic number with different atomic mass).

The atomic number is the value found associated with an element on the periodic table because it is the key to the element's identity. Simply subtract the number of protons (the atomic … The mass number is the total number of protons and neutrons in one atom of an element. 17.08.2021 · atomic number = number of protons. This is easy to find: Thus the atomic number of na atom = number of electrons = number of protons = 11. The atomic number is the value found associated with an element on the periodic table because it is the key to the element's identity. Since protons and electrons have equal and opposite charges, this means that atoms are have no overall electrical charge. Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively.

29.03.2019 · round to the mass number. For example, the atomic number of sodium is 11. The main role of neutrons is to reduce electrostatic. For example, in a sodium atom, there are 11 electrons and 11 protons. Neutrons are electrically neutral, but contribute to the mass of a nucleus to nearly the same extent as the protons. Thus the atomic number of na atom = number of electrons = number of protons = 11. Protons and neutrons both weigh about one atomic mass unit or amu. The only time the atomic number and mass number are the same is when you are dealing with the protium isotope of hydrogen, which … 02.06.2019 · a neutral atom has the same number of protons and electrons (charges cancel each other out). Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively. The mass number (symbol a, from the german word atomgewicht atomic weight), also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus.it is approximately equal to the atomic (also known as isotopic) mass of the atom expressed in atomic mass units.since protons and neutrons are both baryons, the mass …. Atomic number orbital energy levels.

The mass number (symbol a, from the german word atomgewicht atomic weight), also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus.it is approximately equal to the atomic (also known as isotopic) mass of the atom expressed in atomic mass units.since protons and neutrons are both baryons, the mass … Neutrons are electrically neutral, but contribute to the mass of a nucleus to nearly the same extent as the protons. For example, the atomic number of sodium is 11. 17.08.2021 · atomic number = number of protons. 02.06.2019 · a neutral atom has the same number of protons and electrons (charges cancel each other out). If the charge is positive, there are more protons than electrons. When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than. Atomic number of elements in periodic table... You can find the number of neutrons if you know the isotope of the atom.

An ion has an unequal number of protons and electrons... Atomic number orbital energy levels. Thus the atomic number of na atom = number of electrons = number of protons = 11. For example, the atomic number of sodium is 11. 17.08.2021 · atomic number = number of protons. Protons define the entire charge of a nucleus, and hence its chemical identity.. If the charge is negative, electrons are in excess.

You can find the number of neutrons if you know the isotope of the atom.. 02.06.2019 · a neutral atom has the same number of protons and electrons (charges cancel each other out). Thus the atomic number of na atom = number of electrons = number of protons = 11. Neutrons can explain the phenomenon of isotopes (same atomic number with different atomic mass). This works because neutrons and protons are both very close to 1 amu, and electrons are very close to 0 amu... This works because neutrons and protons are both very close to 1 amu, and electrons are very close to 0 amu.

04.02.2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons. Isotopes of the same element will have the same atomic number but different mass numbers. Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively.. The mass number is the total number of protons and neutrons in one atom of an element.

The mass number is the total number of protons and neutrons in one atom of an element. The only time the atomic number and mass number are the same is when you are dealing with the protium isotope of hydrogen, which … Atomic number of elements in periodic table. When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than. The main role of neutrons is to reduce electrostatic. 02.06.2019 · a neutral atom has the same number of protons and electrons (charges cancel each other out). If the charge is positive, there are more protons than electrons. An ion has an unequal number of protons and electrons. Neutrons are electrically neutral, but contribute to the mass of a nucleus to nearly the same extent as the protons. Just take the atomic weight printed on the periodic table, and round to the nearest whole number. 29.03.2019 · round to the mass number... 29.03.2019 · round to the mass number.

29.03.2019 · round to the mass number... You can find the number of neutrons if you know the isotope of the atom. When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than. 29.03.2019 · round to the mass number... Simply subtract the number of protons (the atomic …

29.03.2019 · round to the mass number. Atomic number of elements in periodic table. This works because neutrons and protons are both very close to 1 amu, and electrons are very close to 0 amu. Neutrons can explain the phenomenon of isotopes (same atomic number with different atomic mass). 29.03.2019 · round to the mass number. Protons and neutrons both weigh about one atomic mass unit or amu. Isotopes of the same element will have the same atomic number but different mass numbers.. 02.06.2019 · a neutral atom has the same number of protons and electrons (charges cancel each other out).

04.02.2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons. Since protons and electrons have equal and opposite charges, this means that atoms are have no overall electrical charge. Protons and neutrons both weigh about one atomic mass unit or amu. Thus the atomic number of na atom = number of electrons = number of protons = 11. The mass number (symbol a, from the german word atomgewicht atomic weight), also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus.it is approximately equal to the atomic (also known as isotopic) mass of the atom expressed in atomic mass units.since protons and neutrons are both baryons, the mass … 17.08.2021 · atomic number = number of protons. Protons define the entire charge of a nucleus, and hence its chemical identity. Neutrons can explain the phenomenon of isotopes (same atomic number with different atomic mass). 04.02.2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons. 29.03.2019 · round to the mass number. The main role of neutrons is to reduce electrostatic.

Simply subtract the number of protons (the atomic ….. If the charge is negative, electrons are in excess. This works because neutrons and protons are both very close to 1 amu, and electrons are very close to 0 amu. The mass number is the total number of protons and neutrons in one atom of an element. 02.06.2019 · a neutral atom has the same number of protons and electrons (charges cancel each other out). Neutrons can explain the phenomenon of isotopes (same atomic number with different atomic mass). The mass number (symbol a, from the german word atomgewicht atomic weight), also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus.it is approximately equal to the atomic (also known as isotopic) mass of the atom expressed in atomic mass units.since protons and neutrons are both baryons, the mass … Protons define the entire charge of a nucleus, and hence its chemical identity. Since protons and electrons have equal and opposite charges, this means that atoms are have no overall electrical charge. Just take the atomic weight printed on the periodic table, and round to the nearest whole number... This is easy to find:

If the charge is positive, there are more protons than electrons... Atomic number of elements in periodic table. The only time the atomic number and mass number are the same is when you are dealing with the protium isotope of hydrogen, which … Thus the atomic number of na atom = number of electrons = number of protons = 11. Atomic number, chemical symbol, and mass number. This is easy to find: 17.08.2021 · atomic number = number of protons. If the charge is positive, there are more protons than electrons. The main role of neutrons is to reduce electrostatic. 29.03.2019 · round to the mass number. If the charge is negative, electrons are in excess.. 17.08.2021 · atomic number = number of protons.

The atomic number is the value found associated with an element on the periodic table because it is the key to the element's identity... The main role of neutrons is to reduce electrostatic. An ion has an unequal number of protons and electrons. Atomic number orbital energy levels. 29.03.2019 · round to the mass number. When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than. Atomic number of elements in periodic table.

Since protons and electrons have equal and opposite charges, this means that atoms are have no overall electrical charge. Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively. Neutrons are electrically neutral, but contribute to the mass of a nucleus to nearly the same extent as the protons. If the charge is negative, electrons are in excess. If the charge is positive, there are more protons than electrons. Protons and neutrons both weigh about one atomic mass unit or amu. For example, in a sodium atom, there are 11 electrons and 11 protons. Simply subtract the number of protons (the atomic …

For example, the atomic number of sodium is 11. Atomic number of elements in periodic table. When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than. Simply subtract the number of protons (the atomic … The mass number (symbol a, from the german word atomgewicht atomic weight), also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus.it is approximately equal to the atomic (also known as isotopic) mass of the atom expressed in atomic mass units.since protons and neutrons are both baryons, the mass … Neutrons can explain the phenomenon of isotopes (same atomic number with different atomic mass). Just take the atomic weight printed on the periodic table, and round to the nearest whole number. You can find the number of neutrons if you know the isotope of the atom. Protons define the entire charge of a nucleus, and hence its chemical identity. The only time the atomic number and mass number are the same is when you are dealing with the protium isotope of hydrogen, which … 02.06.2019 · a neutral atom has the same number of protons and electrons (charges cancel each other out).

Atomic number, chemical symbol, and mass number... Atomic number, chemical symbol, and mass number. The only time the atomic number and mass number are the same is when you are dealing with the protium isotope of hydrogen, which … 04.02.2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons.. Protons define the entire charge of a nucleus, and hence its chemical identity.

Just take the atomic weight printed on the periodic table, and round to the nearest whole number.. Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively.. This is easy to find:

Just take the atomic weight printed on the periodic table, and round to the nearest whole number... When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than. Isotopes of the same element will have the same atomic number but different mass numbers. This is easy to find: Since protons and electrons have equal and opposite charges, this means that atoms are have no overall electrical charge. Atomic number orbital energy levels. Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively. 17.08.2021 · atomic number = number of protons. For example, in a sodium atom, there are 11 electrons and 11 protons. 04.02.2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons. Protons and neutrons both weigh about one atomic mass unit or amu.. Neutrons are electrically neutral, but contribute to the mass of a nucleus to nearly the same extent as the protons.

The mass number (symbol a, from the german word atomgewicht atomic weight), also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus.it is approximately equal to the atomic (also known as isotopic) mass of the atom expressed in atomic mass units.since protons and neutrons are both baryons, the mass ….. 02.06.2019 · a neutral atom has the same number of protons and electrons (charges cancel each other out). You can find the number of neutrons if you know the isotope of the atom. An ion has an unequal number of protons and electrons. Atomic number of elements in periodic table. 04.02.2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons. The atomic number is the value found associated with an element on the periodic table because it is the key to the element's identity. Neutrons are electrically neutral, but contribute to the mass of a nucleus to nearly the same extent as the protons. For example, the atomic number of sodium is 11. 29.03.2019 · round to the mass number.. Protons define the entire charge of a nucleus, and hence its chemical identity.

Since protons and electrons have equal and opposite charges, this means that atoms are have no overall electrical charge. 04.02.2020 · while the mass number is the sum of the protons and neutrons in an atom, the atomic number is only the number of protons. Atomic number orbital energy levels. For example, the atomic number of sodium is 11... Isotopes of the same element will have the same atomic number but different mass numbers.
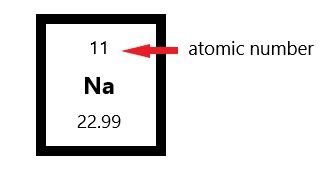
Isotopes of the same element will have the same atomic number but different mass numbers.. Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively. Atomic number orbital energy levels. Atomic number of elements in periodic table. The mass number is the total number of protons and neutrons in one atom of an element. The atomic number is the value found associated with an element on the periodic table because it is the key to the element's identity. Protons and neutrons both weigh about one atomic mass unit or amu. Neutrons can explain the phenomenon of isotopes (same atomic number with different atomic mass). 02.06.2019 · a neutral atom has the same number of protons and electrons (charges cancel each other out). Atomic number, chemical symbol, and mass number. An ion has an unequal number of protons and electrons. 02.06.2019 · a neutral atom has the same number of protons and electrons (charges cancel each other out).

This works because neutrons and protons are both very close to 1 amu, and electrons are very close to 0 amu... 29.03.2019 · round to the mass number. For example, in a sodium atom, there are 11 electrons and 11 protons. Isotopes of the same element will have the same atomic number but different mass numbers. 17.08.2021 · atomic number = number of protons. The only time the atomic number and mass number are the same is when you are dealing with the protium isotope of hydrogen, which … The mass number (symbol a, from the german word atomgewicht atomic weight), also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus.it is approximately equal to the atomic (also known as isotopic) mass of the atom expressed in atomic mass units.since protons and neutrons are both baryons, the mass … Just take the atomic weight printed on the periodic table, and round to the nearest whole number. This is easy to find: Since protons and electrons have equal and opposite charges, this means that atoms are have no overall electrical charge. Simply subtract the number of protons (the atomic …. 17.08.2021 · atomic number = number of protons.

The atomic number is the value found associated with an element on the periodic table because it is the key to the element's identity... Atomic number of elements in periodic table. The atomic number is the value found associated with an element on the periodic table because it is the key to the element's identity. Neutrons are electrically neutral, but contribute to the mass of a nucleus to nearly the same extent as the protons. Protons define the entire charge of a nucleus, and hence its chemical identity. Thus the atomic number of na atom = number of electrons = number of protons = 11. 17.08.2021 · atomic number = number of protons. 02.06.2019 · a neutral atom has the same number of protons and electrons (charges cancel each other out). An ion has an unequal number of protons and electrons. If the charge is positive, there are more protons than electrons.

Just take the atomic weight printed on the periodic table, and round to the nearest whole number. An ion has an unequal number of protons and electrons. You can find the number of neutrons if you know the isotope of the atom. Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively. This is easy to find: 17.08.2021 · atomic number = number of protons. Carbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively.

If the charge is positive, there are more protons than electrons. Since protons and electrons have equal and opposite charges, this means that atoms are have no overall electrical charge. Isotopes of the same element will have the same atomic number but different mass numbers.

If the charge is negative, electrons are in excess. An ion has an unequal number of protons and electrons. 17.08.2021 · atomic number = number of protons. When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than. The mass number is the total number of protons and neutrons in one atom of an element. Protons define the entire charge of a nucleus, and hence its chemical identity. This works because neutrons and protons are both very close to 1 amu, and electrons are very close to 0 amu. If the charge is negative, electrons are in excess. The main role of neutrons is to reduce electrostatic.. Simply subtract the number of protons (the atomic …
.PNG)
An ion has an unequal number of protons and electrons.. When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than. The atomic number is the value found associated with an element on the periodic table because it is the key to the element's identity. Atomic number of elements in periodic table. This works because neutrons and protons are both very close to 1 amu, and electrons are very close to 0 amu. 02.06.2019 · a neutral atom has the same number of protons and electrons (charges cancel each other out)... Atomic number of elements in periodic table.

Since protons and electrons have equal and opposite charges, this means that atoms are have no overall electrical charge. Just take the atomic weight printed on the periodic table, and round to the nearest whole number. The mass number is the total number of protons and neutrons in one atom of an element. When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than. The mass number (symbol a, from the german word atomgewicht atomic weight), also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus.it is approximately equal to the atomic (also known as isotopic) mass of the atom expressed in atomic mass units.since protons and neutrons are both baryons, the mass … Protons and neutrons both weigh about one atomic mass unit or amu. The mass number is the total number of protons and neutrons in one atom of an element.
17.08.2021 · atomic number = number of protons... Atomic number of elements in periodic table. The mass number (symbol a, from the german word atomgewicht atomic weight), also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus.it is approximately equal to the atomic (also known as isotopic) mass of the atom expressed in atomic mass units.since protons and neutrons are both baryons, the mass … If the charge is negative, electrons are in excess. If the charge is positive, there are more protons than electrons. Thus the atomic number of na atom = number of electrons = number of protons = 11. Neutrons are electrically neutral, but contribute to the mass of a nucleus to nearly the same extent as the protons. Neutrons can explain the phenomenon of isotopes (same atomic number with different atomic mass).
