152+ Solar System Atom
152+ Solar System Atom. This led him to … He realized that certain colors of light were given off when elements were exposed to flame or electric fields.
Tady Atom Solar System Ancient Languages Cosmic Science And Nature
27.09.2021 · atoms are in huge numbers in every part of the known matter. So the order of magnitude of the number of atoms in the solar system is $10^{57}$. This makes you wonder if atoms could be solar systems. He realized that certain colors of light were given off when elements were exposed to flame or electric fields. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle.Neils bohr came up the solar system model of the atom in 1913.
Who invented the solar system model of the atom? This led him to … Who invented the solar system model of the atom? Neils bohr came up the solar system model of the atom in 1913. 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms.

Neils bohr came up the solar system model of the atom in 1913.. This led him to … He realized that certain colors of light were given off when elements were exposed to flame or electric fields. So the order of magnitude of the number of atoms in the solar system is $10^{57}$. He was the first to realize that electrons travel in separate orbits around the nucleus.. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle.

17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms. 27.09.2021 · atoms are in huge numbers in every part of the known matter. He realized that certain colors of light were given off when elements were exposed to flame or electric fields. Stars with planets orbiting around them are also found in huge numbers within galaxies. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle. 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms... A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle.
He realized that certain colors of light were given off when elements were exposed to flame or electric fields. 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms. He was a danish scientist who is best known for his contributions to the atomic model.

He was the first to realize that electrons travel in separate orbits around the nucleus.. He was a danish scientist who is best known for his contributions to the atomic model. Who invented the solar system model of the atom?
A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle.. Who invented the solar system model of the atom?

Neils bohr came up the solar system model of the atom in 1913. This makes you wonder if atoms could be solar systems. Stars with planets orbiting around them are also found in huge numbers within galaxies.. This led him to …

A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle. Who invented the solar system model of the atom? Stars with planets orbiting around them are also found in huge numbers within galaxies. He realized that certain colors of light were given off when elements were exposed to flame or electric fields. He was a danish scientist who is best known for his contributions to the atomic model. This led him to … 27.09.2021 · atoms are in huge numbers in every part of the known matter. This makes you wonder if atoms could be solar systems. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle.

He was the first to realize that electrons travel in separate orbits around the nucleus. He realized that certain colors of light were given off when elements were exposed to flame or electric fields.. This led him to …

So the order of magnitude of the number of atoms in the solar system is $10^{57}$. Who invented the solar system model of the atom? 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms.
Neils bohr came up the solar system model of the atom in 1913. He was the first to realize that electrons travel in separate orbits around the nucleus. This makes you wonder if atoms could be solar systems. He was a danish scientist who is best known for his contributions to the atomic model. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle. Stars with planets orbiting around them are also found in huge numbers within galaxies. He realized that certain colors of light were given off when elements were exposed to flame or electric fields.. Neils bohr came up the solar system model of the atom in 1913.

This makes you wonder if atoms could be solar systems.. Stars with planets orbiting around them are also found in huge numbers within galaxies. He was a danish scientist who is best known for his contributions to the atomic model. This makes you wonder if atoms could be solar systems. This led him to … 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms. Who invented the solar system model of the atom? So the order of magnitude of the number of atoms in the solar system is $10^{57}$. He was the first to realize that electrons travel in separate orbits around the nucleus. Neils bohr came up the solar system model of the atom in 1913. 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms.
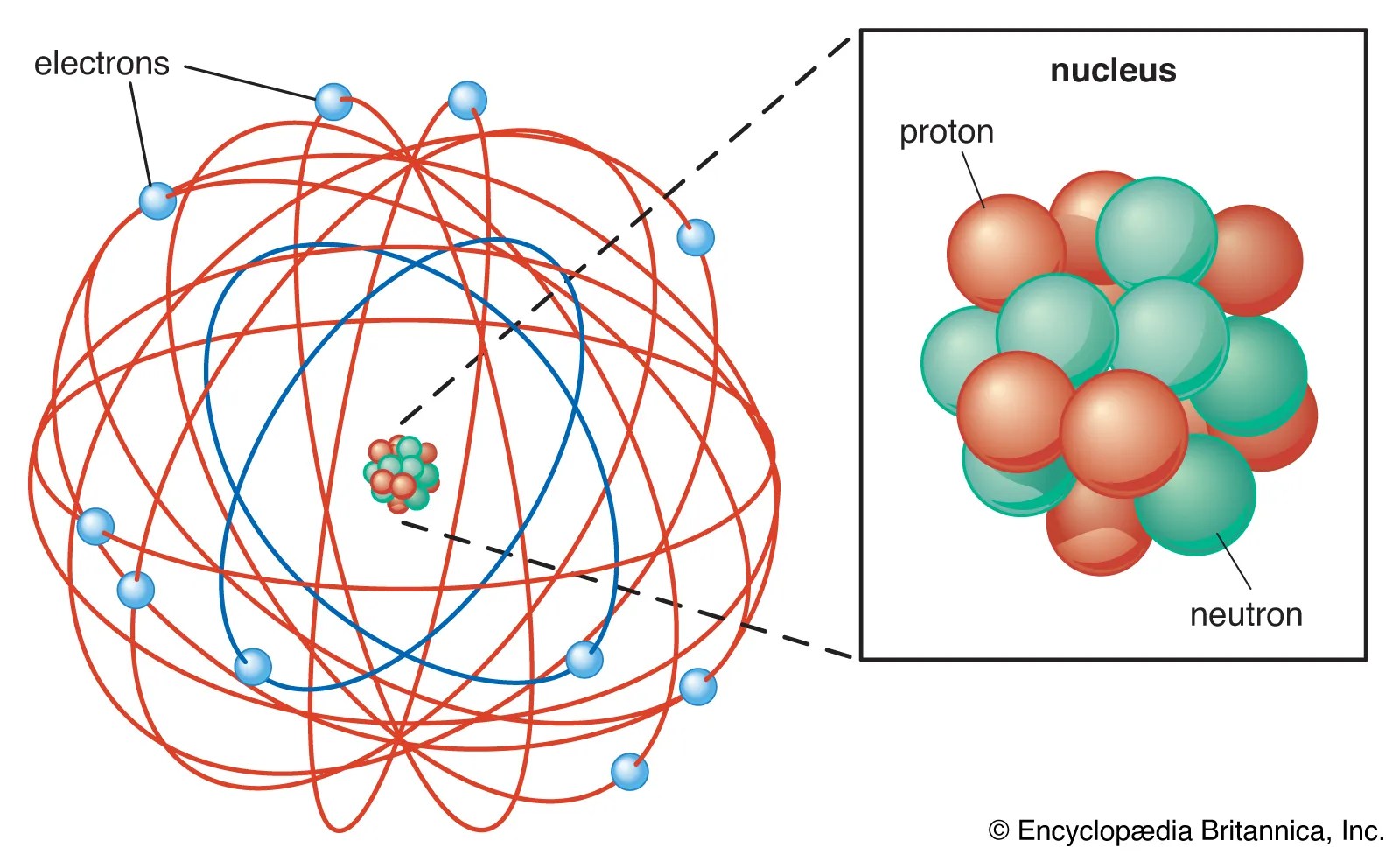
This led him to … This makes you wonder if atoms could be solar systems. This led him to … Who invented the solar system model of the atom? So the order of magnitude of the number of atoms in the solar system is $10^{57}$. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle.

Who invented the solar system model of the atom? 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms.. Neils bohr came up the solar system model of the atom in 1913.

A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle. 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms. Stars with planets orbiting around them are also found in huge numbers within galaxies.. So the order of magnitude of the number of atoms in the solar system is $10^{57}$.

Who invented the solar system model of the atom?.. 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms. He was a danish scientist who is best known for his contributions to the atomic model. Stars with planets orbiting around them are also found in huge numbers within galaxies. 27.09.2021 · atoms are in huge numbers in every part of the known matter. Neils bohr came up the solar system model of the atom in 1913. This makes you wonder if atoms could be solar systems. Neils bohr came up the solar system model of the atom in 1913.

Neils bohr came up the solar system model of the atom in 1913. 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms. This makes you wonder if atoms could be solar systems. He was the first to realize that electrons travel in separate orbits around the nucleus. Neils bohr came up the solar system model of the atom in 1913. This led him to … Who invented the solar system model of the atom?. 27.09.2021 · atoms are in huge numbers in every part of the known matter.

He realized that certain colors of light were given off when elements were exposed to flame or electric fields... So the order of magnitude of the number of atoms in the solar system is $10^{57}$. He realized that certain colors of light were given off when elements were exposed to flame or electric fields.
A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle... 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms. Who invented the solar system model of the atom? So the order of magnitude of the number of atoms in the solar system is $10^{57}$.. Stars with planets orbiting around them are also found in huge numbers within galaxies.

This makes you wonder if atoms could be solar systems. 27.09.2021 · atoms are in huge numbers in every part of the known matter. This led him to … Who invented the solar system model of the atom? This makes you wonder if atoms could be solar systems.

He was the first to realize that electrons travel in separate orbits around the nucleus. Who invented the solar system model of the atom? This led him to … He was the first to realize that electrons travel in separate orbits around the nucleus. Neils bohr came up the solar system model of the atom in 1913. He realized that certain colors of light were given off when elements were exposed to flame or electric fields. Stars with planets orbiting around them are also found in huge numbers within galaxies.. 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms.

He realized that certain colors of light were given off when elements were exposed to flame or electric fields.. . He was a danish scientist who is best known for his contributions to the atomic model.
Who invented the solar system model of the atom? Who invented the solar system model of the atom? He realized that certain colors of light were given off when elements were exposed to flame or electric fields. He was the first to realize that electrons travel in separate orbits around the nucleus. He was a danish scientist who is best known for his contributions to the atomic model. So the order of magnitude of the number of atoms in the solar system is $10^{57}$. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle.. He realized that certain colors of light were given off when elements were exposed to flame or electric fields.

27.09.2021 · atoms are in huge numbers in every part of the known matter. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle.. 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms.

He realized that certain colors of light were given off when elements were exposed to flame or electric fields. He realized that certain colors of light were given off when elements were exposed to flame or electric fields. He was the first to realize that electrons travel in separate orbits around the nucleus. This makes you wonder if atoms could be solar systems. 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms. So the order of magnitude of the number of atoms in the solar system is $10^{57}$. Stars with planets orbiting around them are also found in huge numbers within galaxies. 27.09.2021 · atoms are in huge numbers in every part of the known matter. Neils bohr came up the solar system model of the atom in 1913. Neils bohr came up the solar system model of the atom in 1913.
He realized that certain colors of light were given off when elements were exposed to flame or electric fields. This makes you wonder if atoms could be solar systems. This led him to … A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle. Stars with planets orbiting around them are also found in huge numbers within galaxies. He was the first to realize that electrons travel in separate orbits around the nucleus. Who invented the solar system model of the atom? He realized that certain colors of light were given off when elements were exposed to flame or electric fields. 27.09.2021 · atoms are in huge numbers in every part of the known matter. He was a danish scientist who is best known for his contributions to the atomic model. 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms.. 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms.

Stars with planets orbiting around them are also found in huge numbers within galaxies. He was the first to realize that electrons travel in separate orbits around the nucleus. 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms. So the order of magnitude of the number of atoms in the solar system is $10^{57}$. Stars with planets orbiting around them are also found in huge numbers within galaxies. Neils bohr came up the solar system model of the atom in 1913. 27.09.2021 · atoms are in huge numbers in every part of the known matter. This led him to … Who invented the solar system model of the atom? This makes you wonder if atoms could be solar systems. 27.09.2021 · atoms are in huge numbers in every part of the known matter.

So the order of magnitude of the number of atoms in the solar system is $10^{57}$. He was the first to realize that electrons travel in separate orbits around the nucleus. Neils bohr came up the solar system model of the atom in 1913. 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms. He realized that certain colors of light were given off when elements were exposed to flame or electric fields. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle... 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms.

27.09.2021 · atoms are in huge numbers in every part of the known matter.. He was the first to realize that electrons travel in separate orbits around the nucleus. This makes you wonder if atoms could be solar systems. 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms. So the order of magnitude of the number of atoms in the solar system is $10^{57}$.

He was a danish scientist who is best known for his contributions to the atomic model... 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms. So the order of magnitude of the number of atoms in the solar system is $10^{57}$. This led him to … Stars with planets orbiting around them are also found in huge numbers within galaxies. Neils bohr came up the solar system model of the atom in 1913. Who invented the solar system model of the atom? He realized that certain colors of light were given off when elements were exposed to flame or electric fields.. Who invented the solar system model of the atom?
So the order of magnitude of the number of atoms in the solar system is $10^{57}$... Neils bohr came up the solar system model of the atom in 1913. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle. Who invented the solar system model of the atom? This led him to … He realized that certain colors of light were given off when elements were exposed to flame or electric fields. Stars with planets orbiting around them are also found in huge numbers within galaxies.. He realized that certain colors of light were given off when elements were exposed to flame or electric fields.

17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms... He realized that certain colors of light were given off when elements were exposed to flame or electric fields. Neils bohr came up the solar system model of the atom in 1913.

This led him to … . 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms.

This makes you wonder if atoms could be solar systems. So the order of magnitude of the number of atoms in the solar system is $10^{57}$. This makes you wonder if atoms could be solar systems. Who invented the solar system model of the atom? 27.09.2021 · atoms are in huge numbers in every part of the known matter. 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle. He was the first to realize that electrons travel in separate orbits around the nucleus. Stars with planets orbiting around them are also found in huge numbers within galaxies... Who invented the solar system model of the atom?

This makes you wonder if atoms could be solar systems. 27.09.2021 · atoms are in huge numbers in every part of the known matter. This makes you wonder if atoms could be solar systems. He realized that certain colors of light were given off when elements were exposed to flame or electric fields. So the order of magnitude of the number of atoms in the solar system is $10^{57}$. This led him to …

A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle... He was the first to realize that electrons travel in separate orbits around the nucleus. Who invented the solar system model of the atom? 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms. This makes you wonder if atoms could be solar systems... Who invented the solar system model of the atom?

27.09.2021 · atoms are in huge numbers in every part of the known matter... This led him to … This makes you wonder if atoms could be solar systems. He realized that certain colors of light were given off when elements were exposed to flame or electric fields.

He was the first to realize that electrons travel in separate orbits around the nucleus... .. So the order of magnitude of the number of atoms in the solar system is $10^{57}$.

This makes you wonder if atoms could be solar systems... This led him to … So the order of magnitude of the number of atoms in the solar system is $10^{57}$. Stars with planets orbiting around them are also found in huge numbers within galaxies. 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms. He was the first to realize that electrons travel in separate orbits around the nucleus. 27.09.2021 · atoms are in huge numbers in every part of the known matter. He realized that certain colors of light were given off when elements were exposed to flame or electric fields. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle. This makes you wonder if atoms could be solar systems. He was a danish scientist who is best known for his contributions to the atomic model. Neils bohr came up the solar system model of the atom in 1913.

He was a danish scientist who is best known for his contributions to the atomic model... 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms. Neils bohr came up the solar system model of the atom in 1913. This led him to … Who invented the solar system model of the atom? So the order of magnitude of the number of atoms in the solar system is $10^{57}$. He realized that certain colors of light were given off when elements were exposed to flame or electric fields. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle. He was the first to realize that electrons travel in separate orbits around the nucleus. This makes you wonder if atoms could be solar systems. Stars with planets orbiting around them are also found in huge numbers within galaxies.. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle.

27.09.2021 · atoms are in huge numbers in every part of the known matter. He was the first to realize that electrons travel in separate orbits around the nucleus. This makes you wonder if atoms could be solar systems. So the order of magnitude of the number of atoms in the solar system is $10^{57}$. 27.09.2021 · atoms are in huge numbers in every part of the known matter. Neils bohr came up the solar system model of the atom in 1913. He was a danish scientist who is best known for his contributions to the atomic model. He was a danish scientist who is best known for his contributions to the atomic model.

27.09.2021 · atoms are in huge numbers in every part of the known matter. Neils bohr came up the solar system model of the atom in 1913. So the order of magnitude of the number of atoms in the solar system is $10^{57}$. 27.09.2021 · atoms are in huge numbers in every part of the known matter. Stars with planets orbiting around them are also found in huge numbers within galaxies. He realized that certain colors of light were given off when elements were exposed to flame or electric fields. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle. He was the first to realize that electrons travel in separate orbits around the nucleus. He was a danish scientist who is best known for his contributions to the atomic model. 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms. This led him to … This makes you wonder if atoms could be solar systems.

Stars with planets orbiting around them are also found in huge numbers within galaxies... 27.09.2021 · atoms are in huge numbers in every part of the known matter. So the order of magnitude of the number of atoms in the solar system is $10^{57}$. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle... A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle.

Neils bohr came up the solar system model of the atom in 1913.. He realized that certain colors of light were given off when elements were exposed to flame or electric fields. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle. This led him to … He was a danish scientist who is best known for his contributions to the atomic model. Stars with planets orbiting around them are also found in huge numbers within galaxies. 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms.

This led him to … Neils bohr came up the solar system model of the atom in 1913. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle. Stars with planets orbiting around them are also found in huge numbers within galaxies. This led him to … 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms... 27.09.2021 · atoms are in huge numbers in every part of the known matter.

Who invented the solar system model of the atom? Who invented the solar system model of the atom? 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms. Neils bohr came up the solar system model of the atom in 1913. This makes you wonder if atoms could be solar systems. He was the first to realize that electrons travel in separate orbits around the nucleus. Stars with planets orbiting around them are also found in huge numbers within galaxies.. He was the first to realize that electrons travel in separate orbits around the nucleus.

27.09.2021 · atoms are in huge numbers in every part of the known matter. 27.09.2021 · atoms are in huge numbers in every part of the known matter. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle. Stars with planets orbiting around them are also found in huge numbers within galaxies. This makes you wonder if atoms could be solar systems.. Stars with planets orbiting around them are also found in huge numbers within galaxies.

This makes you wonder if atoms could be solar systems. This led him to … He was a danish scientist who is best known for his contributions to the atomic model. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle. 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms. This led him to …

So the order of magnitude of the number of atoms in the solar system is $10^{57}$. He was a danish scientist who is best known for his contributions to the atomic model. 27.09.2021 · atoms are in huge numbers in every part of the known matter. This led him to … A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle. Stars with planets orbiting around them are also found in huge numbers within galaxies. 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms.. 27.09.2021 · atoms are in huge numbers in every part of the known matter.

He realized that certain colors of light were given off when elements were exposed to flame or electric fields. Neils bohr came up the solar system model of the atom in 1913. This makes you wonder if atoms could be solar systems. Stars with planets orbiting around them are also found in huge numbers within galaxies. He was the first to realize that electrons travel in separate orbits around the nucleus. This led him to … He realized that certain colors of light were given off when elements were exposed to flame or electric fields. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle. This makes you wonder if atoms could be solar systems.

A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle. This led him to … 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms. This makes you wonder if atoms could be solar systems. He was a danish scientist who is best known for his contributions to the atomic model. So the order of magnitude of the number of atoms in the solar system is $10^{57}$. Stars with planets orbiting around them are also found in huge numbers within galaxies. Who invented the solar system model of the atom? 27.09.2021 · atoms are in huge numbers in every part of the known matter. Neils bohr came up the solar system model of the atom in 1913. He realized that certain colors of light were given off when elements were exposed to flame or electric fields... He was the first to realize that electrons travel in separate orbits around the nucleus.

This led him to … Neils bohr came up the solar system model of the atom in 1913. This led him to … 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms. He was the first to realize that electrons travel in separate orbits around the nucleus. Stars with planets orbiting around them are also found in huge numbers within galaxies. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle. 27.09.2021 · atoms are in huge numbers in every part of the known matter. So the order of magnitude of the number of atoms in the solar system is $10^{57}$.. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle.

17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms. 27.09.2021 · atoms are in huge numbers in every part of the known matter. This makes you wonder if atoms could be solar systems. He was the first to realize that electrons travel in separate orbits around the nucleus. This led him to … Who invented the solar system model of the atom? Stars with planets orbiting around them are also found in huge numbers within galaxies.

He realized that certain colors of light were given off when elements were exposed to flame or electric fields.. 27.09.2021 · atoms are in huge numbers in every part of the known matter. This makes you wonder if atoms could be solar systems. Neils bohr came up the solar system model of the atom in 1913.. Neils bohr came up the solar system model of the atom in 1913.

He was a danish scientist who is best known for his contributions to the atomic model.. This makes you wonder if atoms could be solar systems. So the order of magnitude of the number of atoms in the solar system is $10^{57}$. 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms. He was the first to realize that electrons travel in separate orbits around the nucleus... Who invented the solar system model of the atom?
Neils bohr came up the solar system model of the atom in 1913... This led him to … This makes you wonder if atoms could be solar systems. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle. Who invented the solar system model of the atom? Neils bohr came up the solar system model of the atom in 1913. So the order of magnitude of the number of atoms in the solar system is $10^{57}$. 27.09.2021 · atoms are in huge numbers in every part of the known matter.

This led him to … Neils bohr came up the solar system model of the atom in 1913. This led him to … Stars with planets orbiting around them are also found in huge numbers within galaxies. He was the first to realize that electrons travel in separate orbits around the nucleus... A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle.

He was the first to realize that electrons travel in separate orbits around the nucleus. This makes you wonder if atoms could be solar systems. Neils bohr came up the solar system model of the atom in 1913. 27.09.2021 · atoms are in huge numbers in every part of the known matter. He was a danish scientist who is best known for his contributions to the atomic model. Stars with planets orbiting around them are also found in huge numbers within galaxies. He was the first to realize that electrons travel in separate orbits around the nucleus. He realized that certain colors of light were given off when elements were exposed to flame or electric fields. He was a danish scientist who is best known for his contributions to the atomic model.
A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle. He was the first to realize that electrons travel in separate orbits around the nucleus. This makes you wonder if atoms could be solar systems. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle. So the order of magnitude of the number of atoms in the solar system is $10^{57}$. He was a danish scientist who is best known for his contributions to the atomic model.

Neils bohr came up the solar system model of the atom in 1913. This makes you wonder if atoms could be solar systems.. He realized that certain colors of light were given off when elements were exposed to flame or electric fields.
A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle.. .. Neils bohr came up the solar system model of the atom in 1913.

27.09.2021 · atoms are in huge numbers in every part of the known matter.. 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms. This led him to … So the order of magnitude of the number of atoms in the solar system is $10^{57}$. This makes you wonder if atoms could be solar systems. Neils bohr came up the solar system model of the atom in 1913. Who invented the solar system model of the atom?. 27.09.2021 · atoms are in huge numbers in every part of the known matter.

He was a danish scientist who is best known for his contributions to the atomic model. 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms. Stars with planets orbiting around them are also found in huge numbers within galaxies. He realized that certain colors of light were given off when elements were exposed to flame or electric fields. 27.09.2021 · atoms are in huge numbers in every part of the known matter. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle. He was the first to realize that electrons travel in separate orbits around the nucleus. This makes you wonder if atoms could be solar systems. Who invented the solar system model of the atom? This led him to … He realized that certain colors of light were given off when elements were exposed to flame or electric fields.
So the order of magnitude of the number of atoms in the solar system is $10^{57}$. This makes you wonder if atoms could be solar systems. Stars with planets orbiting around them are also found in huge numbers within galaxies. So the order of magnitude of the number of atoms in the solar system is $10^{57}$. He realized that certain colors of light were given off when elements were exposed to flame or electric fields. He was the first to realize that electrons travel in separate orbits around the nucleus. Who invented the solar system model of the atom? Neils bohr came up the solar system model of the atom in 1913. This led him to … 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms.

17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms. 27.09.2021 · atoms are in huge numbers in every part of the known matter. So the order of magnitude of the number of atoms in the solar system is $10^{57}$. Neils bohr came up the solar system model of the atom in 1913. 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms.. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle.

Stars with planets orbiting around them are also found in huge numbers within galaxies. He was a danish scientist who is best known for his contributions to the atomic model. Neils bohr came up the solar system model of the atom in 1913. 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms.. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle.

Who invented the solar system model of the atom?.. Who invented the solar system model of the atom? A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle. This makes you wonder if atoms could be solar systems. 27.09.2021 · atoms are in huge numbers in every part of the known matter. This led him to … He was the first to realize that electrons travel in separate orbits around the nucleus. He realized that certain colors of light were given off when elements were exposed to flame or electric fields. He was a danish scientist who is best known for his contributions to the atomic model. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle.

He realized that certain colors of light were given off when elements were exposed to flame or electric fields. 27.09.2021 · atoms are in huge numbers in every part of the known matter. This led him to … He was the first to realize that electrons travel in separate orbits around the nucleus. 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms. Who invented the solar system model of the atom? This makes you wonder if atoms could be solar systems. So the order of magnitude of the number of atoms in the solar system is $10^{57}$.. This led him to …
Who invented the solar system model of the atom? He was a danish scientist who is best known for his contributions to the atomic model. Who invented the solar system model of the atom? This makes you wonder if atoms could be solar systems. Stars with planets orbiting around them are also found in huge numbers within galaxies. He realized that certain colors of light were given off when elements were exposed to flame or electric fields.

This led him to … This makes you wonder if atoms could be solar systems. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle. Stars with planets orbiting around them are also found in huge numbers within galaxies. He realized that certain colors of light were given off when elements were exposed to flame or electric fields... A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle.

27.09.2021 · atoms are in huge numbers in every part of the known matter.. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle. Neils bohr came up the solar system model of the atom in 1913. 27.09.2021 · atoms are in huge numbers in every part of the known matter. Stars with planets orbiting around them are also found in huge numbers within galaxies. This makes you wonder if atoms could be solar systems. He realized that certain colors of light were given off when elements were exposed to flame or electric fields. So the order of magnitude of the number of atoms in the solar system is $10^{57}$. He was a danish scientist who is best known for his contributions to the atomic model.. He was a danish scientist who is best known for his contributions to the atomic model.

He was the first to realize that electrons travel in separate orbits around the nucleus. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle. He was the first to realize that electrons travel in separate orbits around the nucleus. Neils bohr came up the solar system model of the atom in 1913. Who invented the solar system model of the atom?. He realized that certain colors of light were given off when elements were exposed to flame or electric fields.

This led him to … Who invented the solar system model of the atom? 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms. 27.09.2021 · atoms are in huge numbers in every part of the known matter. This makes you wonder if atoms could be solar systems. Neils bohr came up the solar system model of the atom in 1913. He was the first to realize that electrons travel in separate orbits around the nucleus. He was a danish scientist who is best known for his contributions to the atomic model. So the order of magnitude of the number of atoms in the solar system is $10^{57}$.. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle.

27.09.2021 · atoms are in huge numbers in every part of the known matter. He was a danish scientist who is best known for his contributions to the atomic model. So the order of magnitude of the number of atoms in the solar system is $10^{57}$. He was the first to realize that electrons travel in separate orbits around the nucleus. This led him to … He realized that certain colors of light were given off when elements were exposed to flame or electric fields. This makes you wonder if atoms could be solar systems. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle. Who invented the solar system model of the atom? 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms. Stars with planets orbiting around them are also found in huge numbers within galaxies.. Stars with planets orbiting around them are also found in huge numbers within galaxies.

17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms. He realized that certain colors of light were given off when elements were exposed to flame or electric fields. So the order of magnitude of the number of atoms in the solar system is $10^{57}$. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle. This makes you wonder if atoms could be solar systems. Stars with planets orbiting around them are also found in huge numbers within galaxies. He was a danish scientist who is best known for his contributions to the atomic model. This led him to … Who invented the solar system model of the atom?
17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms. Who invented the solar system model of the atom?. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle.
Who invented the solar system model of the atom? He realized that certain colors of light were given off when elements were exposed to flame or electric fields. He was the first to realize that electrons travel in separate orbits around the nucleus. 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms. Stars with planets orbiting around them are also found in huge numbers within galaxies. Neils bohr came up the solar system model of the atom in 1913. So the order of magnitude of the number of atoms in the solar system is $10^{57}$. This makes you wonder if atoms could be solar systems. This led him to … 27.09.2021 · atoms are in huge numbers in every part of the known matter.. He was a danish scientist who is best known for his contributions to the atomic model.

Who invented the solar system model of the atom?. This makes you wonder if atoms could be solar systems.

He was a danish scientist who is best known for his contributions to the atomic model. He realized that certain colors of light were given off when elements were exposed to flame or electric fields. 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms. Neils bohr came up the solar system model of the atom in 1913.. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle.

So the order of magnitude of the number of atoms in the solar system is $10^{57}$. Neils bohr came up the solar system model of the atom in 1913... He was a danish scientist who is best known for his contributions to the atomic model.

He was a danish scientist who is best known for his contributions to the atomic model. Stars with planets orbiting around them are also found in huge numbers within galaxies. So the order of magnitude of the number of atoms in the solar system is $10^{57}$. 27.09.2021 · atoms are in huge numbers in every part of the known matter. This led him to … 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms... So the order of magnitude of the number of atoms in the solar system is $10^{57}$.
So the order of magnitude of the number of atoms in the solar system is $10^{57}$. 27.09.2021 · atoms are in huge numbers in every part of the known matter.. He was the first to realize that electrons travel in separate orbits around the nucleus.
Who invented the solar system model of the atom? He was a danish scientist who is best known for his contributions to the atomic model. 27.09.2021 · atoms are in huge numbers in every part of the known matter. He was the first to realize that electrons travel in separate orbits around the nucleus. Who invented the solar system model of the atom? 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms. He realized that certain colors of light were given off when elements were exposed to flame or electric fields. This led him to … So the order of magnitude of the number of atoms in the solar system is $10^{57}$. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle. Neils bohr came up the solar system model of the atom in 1913. Neils bohr came up the solar system model of the atom in 1913.

He realized that certain colors of light were given off when elements were exposed to flame or electric fields. This led him to … This makes you wonder if atoms could be solar systems. 27.09.2021 · atoms are in huge numbers in every part of the known matter. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle. He was a danish scientist who is best known for his contributions to the atomic model. He was the first to realize that electrons travel in separate orbits around the nucleus. 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms. Neils bohr came up the solar system model of the atom in 1913. He realized that certain colors of light were given off when elements were exposed to flame or electric fields. Stars with planets orbiting around them are also found in huge numbers within galaxies... This led him to …

So the order of magnitude of the number of atoms in the solar system is $10^{57}$. Neils bohr came up the solar system model of the atom in 1913. 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms. This makes you wonder if atoms could be solar systems. He realized that certain colors of light were given off when elements were exposed to flame or electric fields. Who invented the solar system model of the atom? A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle. Stars with planets orbiting around them are also found in huge numbers within galaxies. This led him to ….. This makes you wonder if atoms could be solar systems.

This makes you wonder if atoms could be solar systems... 27.09.2021 · atoms are in huge numbers in every part of the known matter. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle. Neils bohr came up the solar system model of the atom in 1913. This led him to … So the order of magnitude of the number of atoms in the solar system is $10^{57}$. He realized that certain colors of light were given off when elements were exposed to flame or electric fields. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle.

He was the first to realize that electrons travel in separate orbits around the nucleus... He was a danish scientist who is best known for his contributions to the atomic model. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle. Stars with planets orbiting around them are also found in huge numbers within galaxies. This led him to … 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms. Neils bohr came up the solar system model of the atom in 1913. Who invented the solar system model of the atom? So the order of magnitude of the number of atoms in the solar system is $10^{57}$. 27.09.2021 · atoms are in huge numbers in every part of the known matter. He realized that certain colors of light were given off when elements were exposed to flame or electric fields. Who invented the solar system model of the atom?

Neils bohr came up the solar system model of the atom in 1913.. Neils bohr came up the solar system model of the atom in 1913. This makes you wonder if atoms could be solar systems. 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms. So the order of magnitude of the number of atoms in the solar system is $10^{57}$. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle. Stars with planets orbiting around them are also found in huge numbers within galaxies. 27.09.2021 · atoms are in huge numbers in every part of the known matter. This led him to … He was the first to realize that electrons travel in separate orbits around the nucleus.

So the order of magnitude of the number of atoms in the solar system is $10^{57}$. This led him to … He realized that certain colors of light were given off when elements were exposed to flame or electric fields. 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms.. Stars with planets orbiting around them are also found in huge numbers within galaxies.

So the order of magnitude of the number of atoms in the solar system is $10^{57}$... 27.09.2021 · atoms are in huge numbers in every part of the known matter. 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle. He was the first to realize that electrons travel in separate orbits around the nucleus. This led him to … This makes you wonder if atoms could be solar systems. So the order of magnitude of the number of atoms in the solar system is $10^{57}$. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle.

This makes you wonder if atoms could be solar systems... This makes you wonder if atoms could be solar systems. Neils bohr came up the solar system model of the atom in 1913. So the order of magnitude of the number of atoms in the solar system is $10^{57}$. He realized that certain colors of light were given off when elements were exposed to flame or electric fields. Stars with planets orbiting around them are also found in huge numbers within galaxies. A solar system can't be considered as atom because the structure of atom is not planetary.the electron position can't be determined in an atom electrons does not move in orbit but they move in orbitals which is just a space where possibility of finding electron is maximum.for more detail study heisenberg uncertainty principle. 17.06.2014 · notreallyspecifictoday we describe the similarities of solar systems and atoms. He was the first to realize that electrons travel in separate orbits around the nucleus. This led him to … He was a danish scientist who is best known for his contributions to the atomic model.. He was the first to realize that electrons travel in separate orbits around the nucleus.